Gelation monitoring by (micro)rheological characterization of Pluronic F-127 hydrogels
Related Product
DWS RheoLab™
The DWS RheoLab™ is a contact-free rheometer. It provides access to the sample's viscoelastic properties over an unmatched frequency range and enables the study of textures and microstructures while requiring only small sample volumes.
Abstract
The increasing demand for hydrogel-based advanced functional materials across a broadening diversity of applications drives a need to better understand various hydrogels’ mechanical properties. Typically, bulk rheology is used in this context as it effectively captures low-frequency viscoelastic data. However, inherent limitations in sensitivity and sample handling hinder it from providing a more holistic characterization. Diffusing wave spectroscopy (DWS) complements bulk rheology by precisely measuring the microrheological properties of hydrogels over an extended frequency range (~0.1 rad/s to 1M rad/s) without sample contact. DWS is unaffected by inertia or sensitivity issues in low-viscosity conditions and allows for repeated, non-destructive measurements of low-volume samples that can be sealed. This makes it ideal for studying temperature-dependent processes like sol-gel transitions, providing detailed insights into hydrogel viscoelastic behavior. In this work, the temperature-dependent gelation of the model hydrogel system Pluronic F-127 is characterized by both DWS and bulk rheology and the results are shown and compared. We show that DWS can effectively and accurately capture rheological characteristics across a wide temperature and frequency range.
Introduction
Hydrogels are a versatile class of soft polymeric or particulate materials that form porous, three-dimensional networks in aqueous environments 1. Cross-linked hydrogels offer an attractive alternative for applications like in vivo drug delivery by combining colloidal or monomeric components that swell significantly in water 2. Their reversible assembly and disassembly can be precisely controlled through external stimuli, depending on the design of the oligomers; common triggers include changes in pH, enzymatic activity, and magnetic or thermal signals. Their remarkable water retention, mild processing conditions, ability to entrap substantial quantities of dissolved solutes, and high biocompatibility make hydrogels especially valuable in various biomedical applications, including bone and cartilage regeneration, targeted drug delivery, tissue engineering, biosensors, wound care, and inflammation treatment.
In this work, we use Pluronic Flakes 127 (PF-127), a triblock copolymer widely valued for its thermoresponsive properties and which forms a hydrogel at body temperature above a certain concentration threshold, while remaining a liquid at lower temperatures 3. This unique behavior makes it an ideal model system for studying hydrogel dynamics. PF-127 is often used to enhance the solubility and stability of drugs, offering controlled release and improved bioavailability in treatments such as topical gels, injectable systems, and targeted therapies.
Macroscopic bulk rheology of a hydrogel measured with a standard oscillatory rheometer is a well-established technique for obtaining low-frequency viscoelastic properties. This is useful for assessing material behavior above and below the melting point. However, traditional mechanical rheology has some key limitations, including its sample-invasive nature, low sensitivity to thin gels (tenuous networks), large sample volume requirements, labor-intensive and potentially costly sample preparation, and challenges in detecting microstructural heterogeneities due to the collection of ensemble-averaged information. To address many of these limitations, microrheology using Diffusing Wave Spectroscopy (DWS) provides a powerful and complementary approach 4. This technology enables contact-free, non-invasive, fast, and high-resolution analysis of the viscoelastic properties of hydrogels with low sample volume requirements down to ~150μL. Furthermore, the ability to analyze samples in sealable cuvettes provides a simple sample handling protocol and enables automation and extended shelf-life studies.
In a DWS experiment, the thermally driven nanoscale motion of nanoparticles embedded within the sample is measured using a light scattering-based approach. The measurement yields an ensemble-averaged intensity correlation function used to extract the mean squared displacement (MSD). The MSD can be further transformed into loss (G’’) and storage (G’) moduli. These data provide a wealth of information on the sample microstructure, viscoelasticity, and stability.
Materials & Methods
For the measurements presented in this work, a 20%w/v Pluronic Flake 127 (PF-127) (Merck, Switzerland) hydrogel solution was prepared by dissolving 10 g of PF-127 in 48.2 ml of ice-cold MilliQ water using magnetic stirring. 2 g of Polystyrene tracer particles, 8 %w/w with 460-nm diameter (LS Instruments, Switzerland) were added to the transparent mixture to achieve the turbidity necessary for DWS measurements. A homogeneous solution was achieved by additional magnetic stirring. The sample was stored at 4 °C until the start of the acquisition.
DWS Microrheology
We performed microrheology measurements using a DWS RheoLab III by LS Instruments. 1.5 ml of the 20%w/v PF-127 solution was loaded into a 10 mm x 10 mm x 45mm DWS cuvette, sealed with a Teflon stopper, and placed inside the instrument. An automated measurement was programmed, with a heating ramp from 10 °C to 40 °C in steps of 3 °C and a waiting time of 5 minutes at each temperature to ensure thermal equilibrium. Isothermal measurements were then performed with 5 repetitions of 60 seconds in ‘normal DWS’ and 60 seconds in ‘Echo DWS’ modes, both in transmission geometry.
Bulk Rheology
We performed oscillatory shear measurements on PF-127 using a Physica MCR 501 bulk rheometer by Anton Paar, equipped with a Couette (concentric cylinder) geometry. The sample volume was 15 ml. Frequency sweeps from 1 rad/s to 100 rad/s and temperature ramps from 10 °C to 40 °C in steps of 3 °C were performed at 1% strain. The temperature ramp was performed at a fixed frequency of 1 rad/s, averaging over 5 minutes. An in-house customized vapor trap was used to minimize evaporation during the measurement. Note that some discrepancies between DWS microrheology measurements and bulk rheology measurements can occur due to differences in how each technology performs the measurements; for more detailed explanations and reading, please refer to the given references 5–7
Results & Discussion
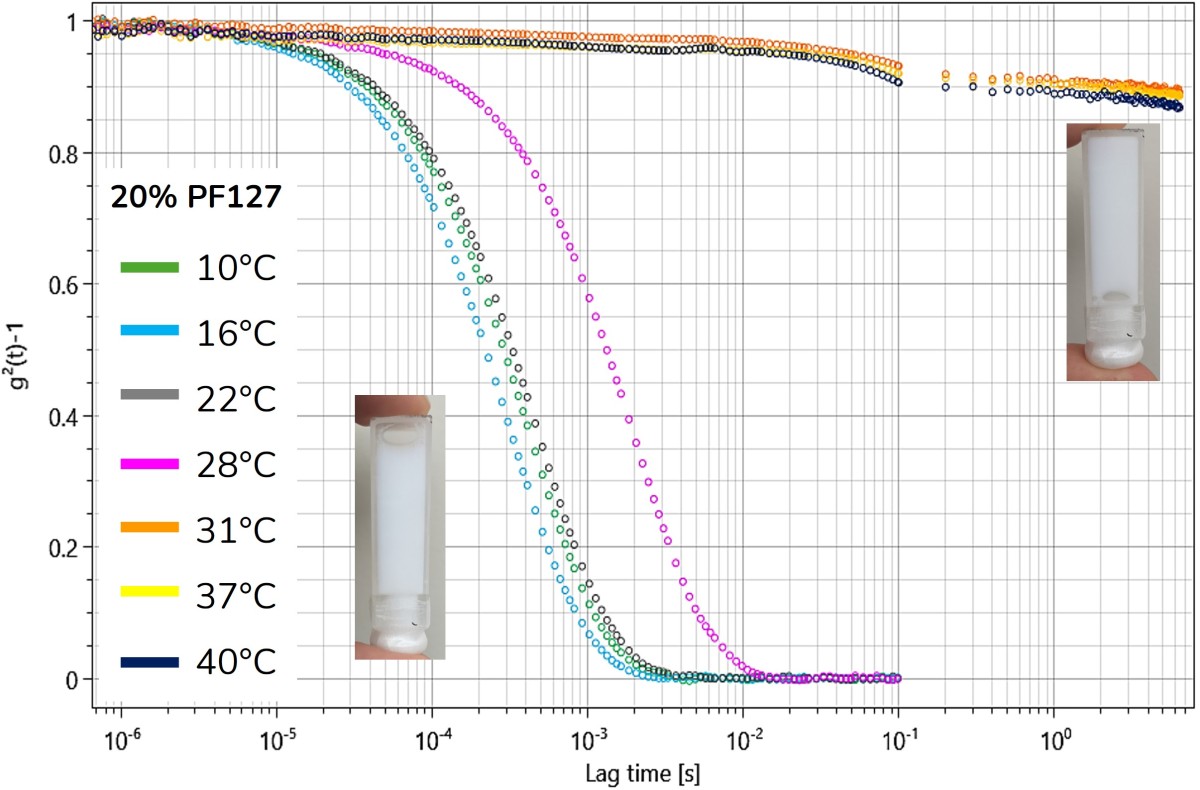
Figure 1. Correlation functions of a 20%w/v PF-127 solution during heating from 10 °C to 40 °C in DWS. Photographs of the sample illustrate the liquid state at lower temperatures and the gelled sample at higher temperatures.
Figure 1 presents the results from diffusing wave spectroscopy (DWS) measurements, displaying a representative intensity autocorrelation function of a 20%w/v PF-127 solution for selected temperatures in the range 10 °C - 40 °C. Initially, a slight reduction in decay time is observed, indicating increased particle mobility at temperatures between 10 °C and 16 °C. This is followed by a marked increase in relaxation time from 22 °C to 28 °C, beyond which the correlation functions no longer decay to zero, indicating restricted motion of the tracer particles and suggesting a transition to a more structured or gel-like state.

Figure 2. Mean-squared displacement (MSD) curves of a 20%w/v PF-127 solution during heating from 10 °C to 40 °C in DWS.
The mean-squared displacement (MSD) data in Figure 2 further corroborates the observations from the correlation functions. The linear slope of approximately 1 of the green and blue curves at low temperatures confirms that the sample is in a liquid, nearly Newtonian state. With increasing temperature towards 28 °C, the MSD curves are positioned below the green curve, indicating increased viscoelasticity, a signature of the onset of gelation. Additionally, a deviation from a linear slope indicates a change in the sample behavior deviating from Brownian diffusion at 28 °C (pink curve). Above 30 °C (orange and yellow), the curves display a clear plateau at long lag times, signifying that the PF-127 solution is kinetically arrested and fully gelled.
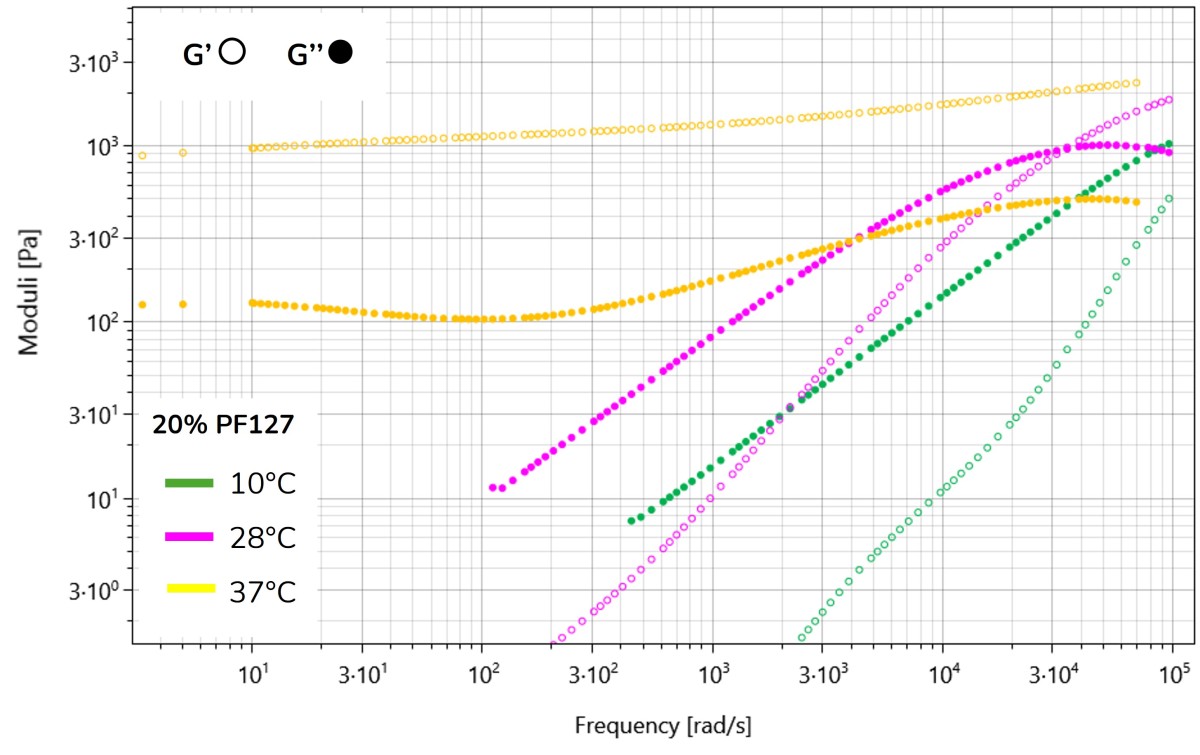
Figure 3. Averaged G’ and G’’ curves of the 20% w/v PF-127 solution at 10 °C, 28 °C, and 37 °C measured via DWS.
Examining the G′ and G′′ curves in Figure 3 over the specified temperature range reveals distinct transitions in sample behavior. At 10 °C, G′′ increases linearly with frequency, indicating liquid-like behavior. At 28 °C, both G′ and G′′ ′ increase with frequency, with G′′ higher than G′ before a cross-over can be seen, indicating that the gel structure has started to form. At 37 °C, solid-like behavior is observed, with G′ remaining above G′′ and a plateau spanning the entire frequency range. The DWS measurements effectively captured the gelation process of the 20%w/v PF-127 solution, accurately identifying the start of gelation (sol-gel transition) above 28 °C 3.
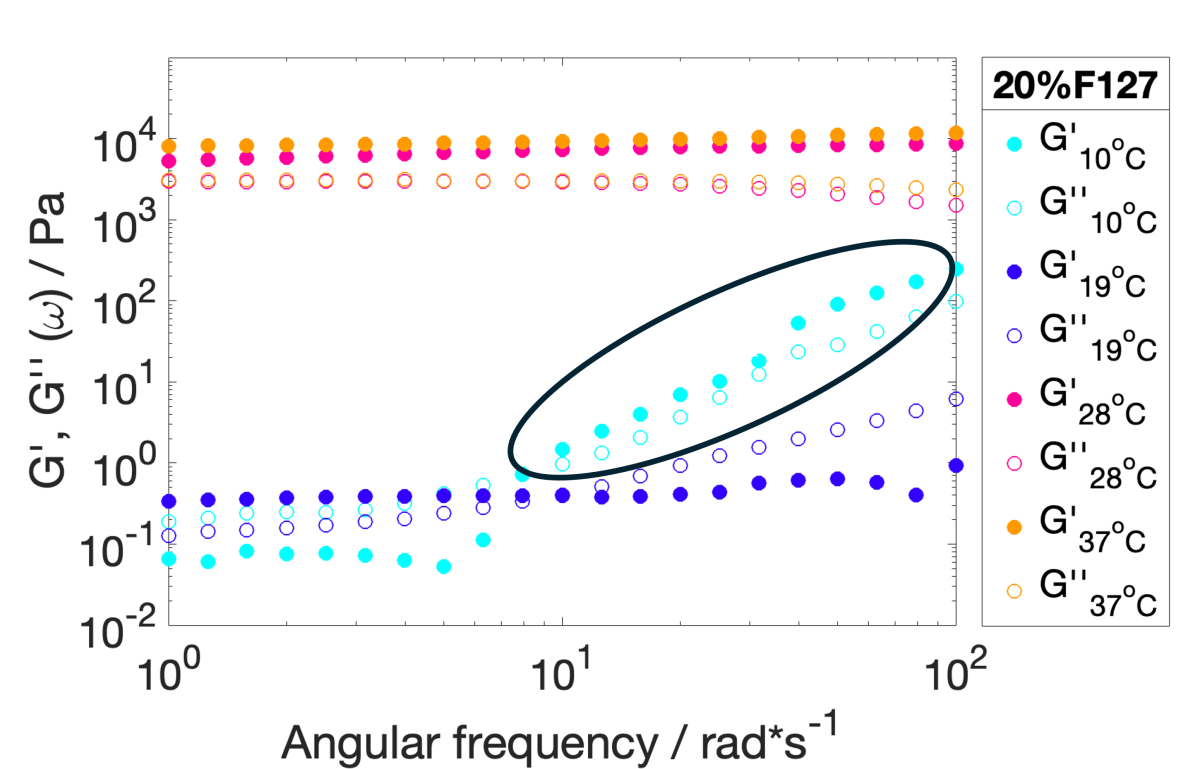
Figure 4. G’ and G’’ curves of the 20%w/v PF-127 solution at 10 °C, 28 °C, and 37 °C measured via bulk rheometry. The black ellipse indicates data points where inaccuracies arise due to inertia issues related to low viscoelasticity of the sample at low temperatures.
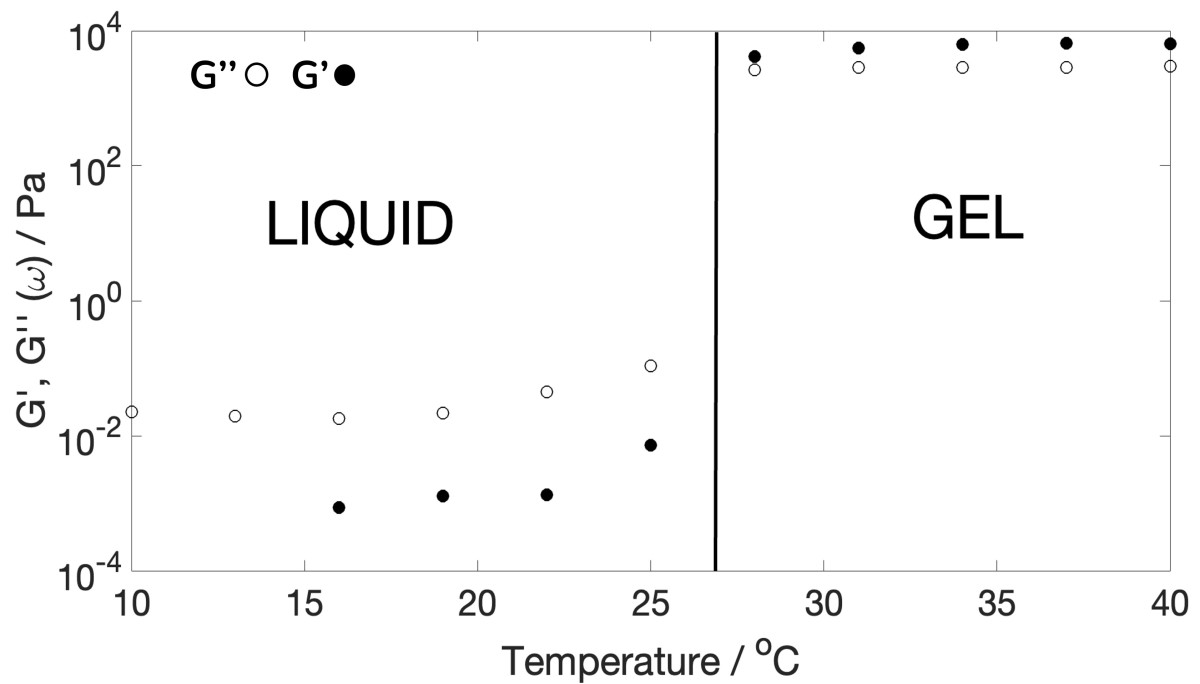
Figure 5. Plot to extract the sol-gel transition for a 20%w/v PF-127 solution at 1% strain and 1 rad/s with bulk rheometry.
Bulk rheometry measurements were conducted on the same solution using the same temperature ramp as the DWS measurements. At lower temperatures, mechanical rheometry faced challenges in obtaining accurate data at higher frequencies, primarily due to inertial effects caused by the low viscoelasticity of the PF-127 solution below 20 °C. However, for temperatures above 20 °C, both bulk rheometry (Figures 4 and 5) and Diffusing Wave Spectroscopy (DWS) results consistently revealed a sol-gel transition occurring just below 30 °C.
Conclusion
The gelation process of the model hydrogel system PF-127 was effectively monitored, and the sol-gel transition was accurately characterized using Diffusing Wave Spectroscopy (DWS). This method facilitated rapid measurements on small sample volumes within sealable cuvettes, which minimized sample consumption and enabled repeated measurements over extended periods. As a complementary technique to bulk rheology, the contact-free nature of DWS ensured reliable analysis of samples with low viscoelastic properties, demonstrating its efficiency in studying the sol-gel transition in PF-127.
Acknowledgments
We gratefully acknowledge Dr. Iliya D. Stoev for his substantial contributions to this work.
References
(1) Xing, Z.; Caciagli, A.; Cao, T.; Stoev, I.; Zupkauskas, M.; O’Neill, T.; Wenzel, T.; Lamboll, R.; Liu, D.; Eiser, E. Microrheology of DNA Hydrogels. 2017.
(2) Kesharwani, P.; Bisht, A.; Alexander, A.; Dave, V.; Sharma, S. Biomedical Applications of Hydrogels in Drug Delivery System: An Update. Journal of Drug Delivery Science and Technology. Elsevier December 1, 2021.
(3) Prud’homme, R. K.; Wu, G.; Schneider, D. K. Structure and Rheology Studies of Poly(Oxyethylene-Oxypropylene-Oxyethylene) Aqueous Solution. Langmuir 1996.
(4) Kim, H. S.; Şenbil, N.; Zhang, C.; Scheffold, F.; Mason, T. G. Diffusing Wave Microrheology of Highly Scattering Concentrated Monodisperse Emulsions. Proc. Natl. Acad. Sci. U. S. A. 2019.
(5) Xu, J.; Boddu, V. M.; Kenar, J. A. Micro-Heterogeneity and Microrheological Properties of Cellulose-Based Hydrogels Studied by Diffusing Wave Spectroscopy (DWS). Cellul. Chem. Technol. 2024.
(6) Xue, X.; Miao, X.; Liu, J.; Ding, Y.; Zhang, Y.; Sun, Y.; Huang, W.; Jiang, Q.; Jiang, B.; Komarneni, S. Investigating the PH-Dependence of Gelation Process in Chitosan-Glutaraldehyde Hydrogels with Diffusing Wave Spectroscopy. Polymer (Guildf). 2025.
(7) Vanin, A. P.; Camassola, M.; Eiser, E.; Stokke, B. T. Characterization of the Polysaccharide Schizophyllan and Schizophyllan–Chitosan Hydrogel Formation by Diffusing-Wave Spectroscopy. Carbohydr. Polym. 2025.
