Aging of an Emulsion Followed by DWS Sizing - Ostwald Ripening Mechanism
Introduction
Diffusing Wave Spectroscopy (DWS) is a modern light scattering technique that probes the motion of particles in turbid colloidal media, such as concentrated suspensions, emulsions, and foams [1]. From the particle motion, the rheological properties of the surrounding medium can be obtained via the so-called microrheology approach [2], as well as information about particle size [1,3-4]. DWS can, indeed, provide the precise mean hydrodynamic radius Rh of solid particles or liquid droplets in highly concentrated suspensions or emulsions (cf. [3] and our technical note Particle Sizing using DWS). The diameter of bubbles in foams can also be estimated [4]. DWS can, consequently, monitor the size evolution of particles in aging dispersions [4,5]. Ostwald ripening is a common aging process, which typically arises in the early stages of the emulsion life as a result of the partial solubility of the dispersed phase in the continuous phase [6]. Due to interfacial tension that makes smaller droplets less stable than bigger droplets, the dispersed phase subsequently diffuses from the smaller to larger droplets through the continuous phase. This results in an increase in the mean droplet size with time (Figure 1).
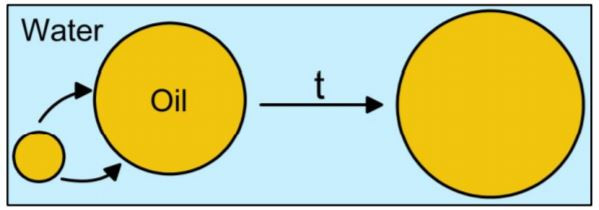
Figure 1. Ostwald ripening mechanism. Oil (dispersed phase) diffuses through water (continuous phase) from smaller to bigger droplets.
When the process is diffusion-controlled, the radius of the droplets increases with t1/3 [7,8]. In this application note, we monitor the time-evolution of the mean hydrodynamic radius Rh of oil droplets dispersed in water using the DWS RheoLab. After an initial transient period, we observe that diffusion-controlled Ostwald ripening becomes the mechanism that determines the aging rate of the emulsion.
Sample Preparation
Dodecane was emulsified at a volume fraction of 13% in water containing 80 mmol.L-1 of sodium dodecyl sulfate using an ultrasound probe (UP200H, Dr. Hielscher, Germany) for 10 min at full power, with pulses of 0.3 s every second. Subsequently, the emulsion was filled into a 10 mm thick glass cuvette (Figure 2), which was loaded in the DWS RheoLab, set to 22°C. DWS measurements of the hydrodynamic radius Rh were performed every 5 min. The sample remained in the instrument for the entire duration of the experiment.

Figure 2. Glass cuvette containing the oil-in-water emulsion under interest.
Results and discussion
Figure 3 shows the time-evolution of the mean hydrodynamic radius Rh obtained with the DWS RheoLab. For the sake of clarity, the horizontal scale is displayed in t1/3, and the reported values of Rh are block-averaged over intervals Δt1/3= 2.5 s1/3. The vertical error bars indicate the standard deviations associated with the block average. The choice of the block size did not alter the results.
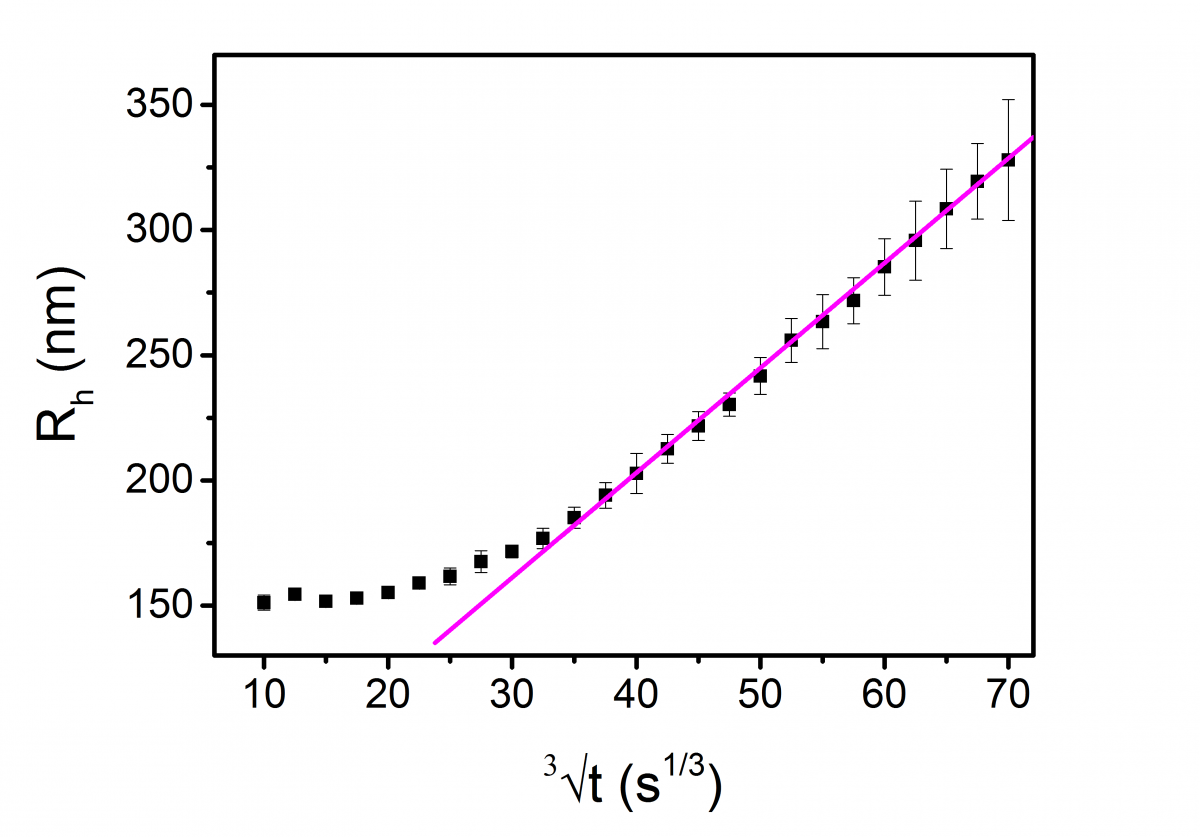
Figure 3. Time-evolution of the hydrodynamic radius Rh of the oil droplets within a concentrated emulsion, as measured by the DWS RheoLab. The line is a fit of the experimental points.
Immediately after emulsification, the first value measured by DWS was Rh= 152 nm. We note that the same value was obtained by dynamic light scattering, using a 3D LS Spectrometer, on a volume of emulsion that had, immediately following emulsification, been diluted with the same aqueous phase composing the original emulsion. After a transient period of eight hours, Rh starts to grow linearly with t1/3. This behavior, which was observed for the duration of four days, is consistent with a diffusion-controlled Ostwald ripening process, as described above.
Conclusion
Using the DWS RheoLab, we have been able to accurately monitor the time-evolution of the mean hydrodynamic radius Rh of oil droplets dispersed in a concentrated emulsion. We have further demonstrated that, after an initial transient period, diffusion-controlled Ostwald ripening becomes the mechanism that determines the aging rate of the emulsion.
The results highlight that the DWS RheoLab is a valuable tool for the characterization of aging and stability of emulsions.
References
[1] D.A. Weitz, and D.J. Pine, Diffusing-Wave Spectroscopy. In Dynamic Light Scattering; Brown, W., Ed.; Oxford University Press: New York, 652 (1993).
[2] T.G. Mason, D.A. Weitz, Optical Measurements of Frequency-Dependent Linear Viscoelastic Moduli of Complex Fluids, Physical Review Letters 74, 1250 (1995).
[3] F. Scheffold, Particle sizing with Diffusing Wave Spectroscopy, Journal of Dispersion Science and Technology 23, 591 (2002).
[4] D.J. Durian, D.A. Weitz, and D.J. Pine, Scaling behavior in shaving cream, Physical Review A 44, R7902(R) (1991).
[5] Y. Hemar, and D. S. Horne, A diffusing-wave spectroscopy study of the kinetics of Ostwald ripening in protein- stabilized oil/water emulsions, Colloids and Surfaces B 12, 239 (1999).
[6] V. Schmitt, and F. Leal-Calderon, Measurement of the coalescence frequency in surfactant-stabilized concentrated emulsions, Europhysics Letters 67, 662 (2004).
[7] I.M. Lifshitz, and V.V. Slyozov, Kinetics of diffusive decomposition of supersaturated solid solutions, Journal of Experimental and Theoretical Physics 35, 331 (1959).
[8] C. Wagner, Theory of the aging of precipitates by dissolution-reprecipitation (Ostwald ripening), Zeitschrift für Elektrochemie 65, 581 (1961).
