Cosmetic Formulation with DWS Microrheology
Related Product
DWS RheoLab™
The DWS RheoLab™ is a contact-free rheometer. It provides access to the sample's viscoelastic properties over an unmatched frequency range and enables the study of textures and microstructures while requiring only small sample volumes.
Abstract
Optical microrheological techniques are gaining increasing interest as a technique of choice to extend the frequency range to high frequencies and gain insight into the rheology and dynamics of wormlike micelles and other complex fluids systems [1, 2-8]. It has been shown [9] that the high-frequency response of entangled wormlike micelle can be utilized to extract out some of the key microstructural parameters of wormlike micelles such as the persistence length and contour length. In this study, Diffusing Wave Spectroscopy (DWS) is utilized to explore the evolution in the wormlike micellar structure in sodium laureth sulfate (SLES) / cocamidopropyl betaine (CAPB) system as a rhamnolipid biosurfactant (mono/di-rhamnolipids mixture, CCB) is introduced. A systematic evaluation of the evolution of the rheology and dynamics is explored on changing the surfactant to biosurfactant ratio, and changing formulation conditions, such as rhamnolipids concentration, salt concentration, and pH values. The primary goal of this study is to provide some formulation guidance on rheology impacts and modifications for the cosmetic and personal care industry.
Sample Preparation and Measurements
Effect of Ratio
Figure 1 shows Maxwellian type response in SLES, SLES/CAPB and SLES/CAPB/CCB system. However, much weaker entanglement and potential shortening of micelles seems to be taking place on addition of CCB. As shown in the table below, the SLES/CAPB system formed long wormlike micelle with a contour length of 445.8 nm, and the short entanglement length and persistence length
indicated the high degree of entanglement and flexibility of the wormlike micelle. With 2 wt% addition of CCB, contour length
of the wormlike micelle reduced to about 20% of its original length while the persistence length did not decrease significantly. This indicates the long flexible wormlike micelles in SLES/CAPB morphed into shorter, rigid rods. This structure change is potentially the fundamental reason for the rheological phenomenon we observed in SLES/CAPB/CCB systems, which is a reduction in viscosity with increasing CCB addition.
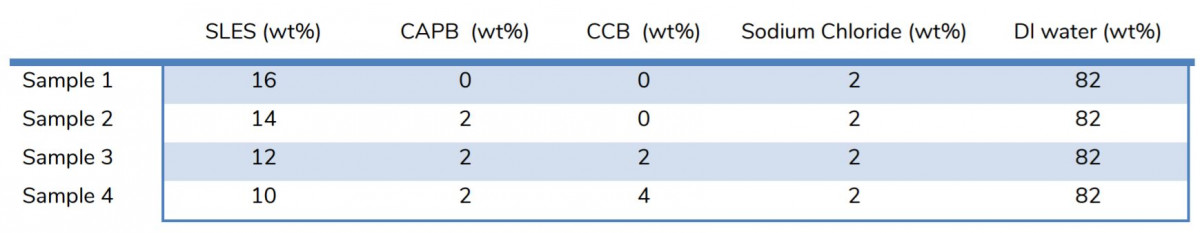
Table 1. Samples composition where the total surfactant is fixed at 16 wt%.
Effect of Adding Salt
The loss of viscosity on addition of CCB is not favorable for personal care products. One of the most common methods to rebuild system viscosity is by adding salts such as sodium chloride to the system, since sodium chloride tends to screen charges between surfactant head groups and further promotes the growth of wormlike micelles. In this manner viscosity of the SLES/CAPB/CCB can be built up to a certain extent. However, viscosity decreases significantly when salt concentration ≥ 4 wt%. This is possibly due to shortening of the micelles or branching. The structural parameters extracted from the DWS in table 2 corresponded to the viscosity change in the system. When salt concentration is 4 wt%, contour length of the micelles is the longest whereas the entanglement length and persistence length is in relation shorter than for other samples. A decrease of micellar contour length was found when salt concentration becomes greater than 4 wt%. Persistence length also increases in this case. These data indicate the potential shortening of the micelles with higher rigidity.
Effect of pH
In the same SLES/CAPB/CCB system, pH impacts the system’s rheological response significantly. Due to the zwitterionic nature of CAPB, it become more cationic at lower pH while rhamnolipids become more nonionic. SLES still maintains its anionic nature at low pH and this promotes the interaction between CAPB and SLES. At lower pH, overall loss and storage modulus exhibits higher values while longer relaxation times were observed. Increase in micelles contour length under lower pH conditions was confirmed by the DWS data.
Results and Discussion
Effect of Ratio
Figure 1 shows Maxwellian type response in SLES, SLES/CAPB and SLES/CAPB/CCB system. However, much weaker entanglement and potential shortening of micelles seems to be taking place on addition of CCB. As shown in the table below, the SLES/CAPB system formed long wormlike micelle with a contour length of 445.8 nm, and the short entanglement length 𝑙𝑒 and persistence length 𝑙𝑝 indicated the high degree of entanglement and flexibility of the wormlike micelle. With 2 wt% addition of CCB, contour length 𝐿̅ of the wormlike micelle reduced to about 20% of its original length while the persistence length did not decrease significantly. This indicates the long flexible wormlike micelles in SLES/CAPB morphed into shorter, rigid rods. This structure change is potentially the fundamental reason for the rheological phenomenon we observed in SLES/CAPB/CCB systems, which is a reduction in viscosity with increasing CCB addition.

Figure 1: Microrheological responses for sample 1,2 and 3 over the entire frequency. Table shows size data extracted from the high frequency G’, G” result, where 𝐿̅ is the contour length, 𝑙𝑒 is the entanglement length and 𝑙𝑝 is the persistence length of the wormlike micelle.
Effect of Adding Salt
The loss of viscosity on addition of CCB is not favorable for personal care products. One of the most common methods to rebuild system viscosity is by adding salts such as sodium chloride to the system, since sodium chloride tends to screen charges between surfactant head groups and further promotes the growth of wormlike micelles. In this manner viscosity of the SLES/CAPB/CCB can be built up to a certain extent. However, viscosity decreases significantly when salt concentration ≥ 4 wt%. This is possibly due to shortening of the micelles or branching. The structural parameters extracted from the DWS in table 2 corresponded to the viscosity change in the system. When salt concentration is 4 wt%, contour length of the micelles is the longest whereas the entanglement length and persistence length is in relation shorter than for other samples. A decrease of micellar contour length was found when salt concentration becomes greater than 4 wt%. Persistence length also increases in this case. These data indicate the potential shortening of the micelles with higher rigidity.

Table 2: Salt effect on the SLES/CAPB/CCB system’s contour length, entanglement length and persistence length.
Effect of pH
In the same SLES/CAPB/CCB system, pH impacts the system’s rheological response significantly. Due to the zwitterionic nature of CAPB, it become more cationic at lower pH while rhamnolipids become more nonionic. SLES still maintains its anionic nature at low pH and this promotes the interaction between CAPB and SLES. At lower pH, overall loss and storage modulus exhibits higher values while longer relaxation times were observed. Increase in micelles contour length under lower pH conditions was confirmed by the DWS data.
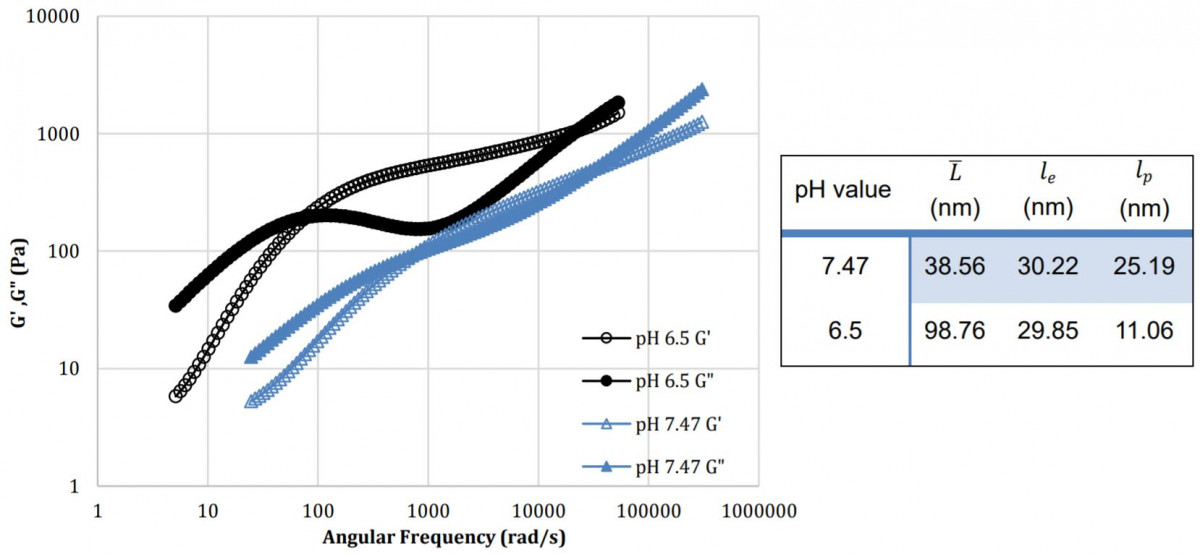
Figure 2: pH influence on the 10wt% SLES, 2wt% CAPB and 4wt% CCB system where salt concentration is fixed at 2wt%. Table shows size data for the surfactant system at different pH value.
Conclusions
This preliminary rheological and microstructure study of the complex biosurfactant/surfactants ternary system utilized DWS microrheology to reveal the effect of formulation conditions on the mixtures rheological responses. This unique characterization technique extended the frequency range which enables us to extract key microstructural parameters of wormlike micelles. All measurements were conducted with a DWS RheoLab. A more comprehensive account of this study has been published in reference [1]
References
[1] Xu L, Amin S. Microrheological study of ternary surfactant‐biosurfactant mixtures. International Journal of Cosmetic Science. 2019 May 17.
[2] Amin S, Blake S, Kennel R, Lewis E. Revealing new structural insights from surfactant micelles through DLS, microrheology and Raman spectroscopy. Materials. 2015; 8(6):3754-66.
[3] Oelschlaeger C, Schopferer M, Scheffold F, Willenbacher N. Linear-to-branched micelles transition: A rheometry and diffusing wave spectroscopy (DWS) study. Langmuir. 2008 Dec 16; 25(2):716-23.
[4] Li Z, Dai L, Wang D, Mao L, Gao Y. Stabilization and rheology of concentrated emulsions using the natural emulsifiers quillaja saponins and rhamnolipids. Journal of Agricultural and Food Chemistry. 2018 Mar 29; 66(15):3922-9.
[5] Mason TG, Weitz DA. Optical measurements of frequency-dependent linear viscoelastic moduli of complex fluids. Physical Review Letters. 1995 Feb 13; 74(7):1250.
[6] van Zanten JH, Amin S, Abdala AA. Brownian motion of colloidal spheres in aqueous PEO solutions. Macromolecules. 2004 May 18; 37(10):3874-80.
[7] Amin S, Rega CA, Jankevics H. Detection of viscoelasticity in aggregating dilute protein solutions through dynamic light scattering-based optical microrheology. Rheologica Acta. 2012 Apr 1; 51(4):329-42.
[8] Abdala AA, Amin S, van Zanten JH, Khan SA. Tracer microrheology study of a hydrophobically modified comblike associative polymer. Langmuir. 2015 Mar 24; 31(13):3944-51.
[9] Willenbacher N, Oelschlaeger C, Schopferer M, Fischer P, Cardinaux F, Scheffold F. Broad bandwidth optical and mechanical rheometry of wormlike micelle solutions. Physical Review Letters. 2007 Aug 10; 99(6):068302.
