Emulsions Characterized by Diffusing Wave Spectroscopy
Related Product
DWS RheoLab™
The DWS RheoLab™ is a contact-free rheometer. It provides access to the sample's viscoelastic properties over an unmatched frequency range and enables the study of textures and microstructures while requiring only small sample volumes.
Introduction
Diffusing Wave Spectroscopy (DWS) is a powerful optical technique specifically suited to study the rheological properties of turbid samples. The method is based on the analysis of the fluctuations of coherent laser light that is scattered multiple times within a sample [1,2]. The DWS RheoLab from LS Instruments is a versatile tool to conduct such measurements.
DWS requires well-defined scattering objects embedded in the sample. They can naturally be part of the sample or added for the purpose of enabling DWS measurements on otherwise transparent samples. In both cases, they are termed tracer particles and are ideally monodisperse with high optical contrast. However, in emulsions such as lotions, creams, or milk, the scattering objects are liquid droplets instead of solid particles, and the droplet size distribution of emulsions often shows considerable polydispersity.
The purpose of this application note is to demonstrate that emulsion droplets can nonetheless act as natural tracers to probe the rheological properties of emulsions using DWS. We apply this methodology to a model system which is a 20 vol.% oil-in-water emulsion with xanthan in the continuous phase as a viscosity modifier. Furthermore, we compare measurements of the viscoelastic properties of the emulsion obtained by DWS microrheology and mechanical rheology.
Sample preparation
The continuous phase is prepared by dissolving 0.55 wt.% xanthan and 0.3 wt.% Cosmocil as an antimicrobial agent in distilled water. This solution is heated for 3 hours to 85°C to avoid sensitivity to thermal history [3] and subsequently stirred for 48 hours. The emulsion is prepared by heating 164 g of the continuous phase to 50°C and adding 2.1 g Tween 80. During homogenization, 34 g of almond oil (density: 0.91 g/mL) is added and then subsequently homogenized further for 12 minutes. All measurements are performed at a temperature of 25°C and 14 days after preparation of the samples.
Characterization of the Emulsion
The emulsion is first investigated by confocal microscopy to access the droplet size. Figure 1 shows an image of the emulsion stained with lipophilic Nile red. The oil droplets are nicely dispersed and fully arrested in the xanthan matrix of the continuous phase. The inset shows the corresponding droplet size distribution.

Figure 1. Confocal image of the emulsion stained with Nile red. The inset shows the corresponding droplet size distribution.
DWS on the emulsion is performed in cuvettes with thickness L=2 mm in transmission geometry. For calibration, a dispersion in water of 1.0 wt.% PS particles with a diameter of 980 nm is used. The measured value for the transport means free path l* of the emulsion is 226 µm. For the tracer particle diameter, we use a value of 1.2 µm, which corresponds to the main peak in the inset of Figure 1. Mechanical rheology is performed on a Malvern Bohlin Gemini rheometer equipped with sand blasted plane-cone geometry (60 mm, 2°) and solvent trap. The storage G’ and loss moduli G’’ are measured in oscillation mode with a constant amplitude of 0.1, which is found to be in the linear viscoelastic range by performing an amplitude sweep at 1 Hz. Figure 2 compares the values for G’ and G’’ measured by DWS microrheology and mechanical rheology. In the overlapping frequency range, the data sets show good agreement.
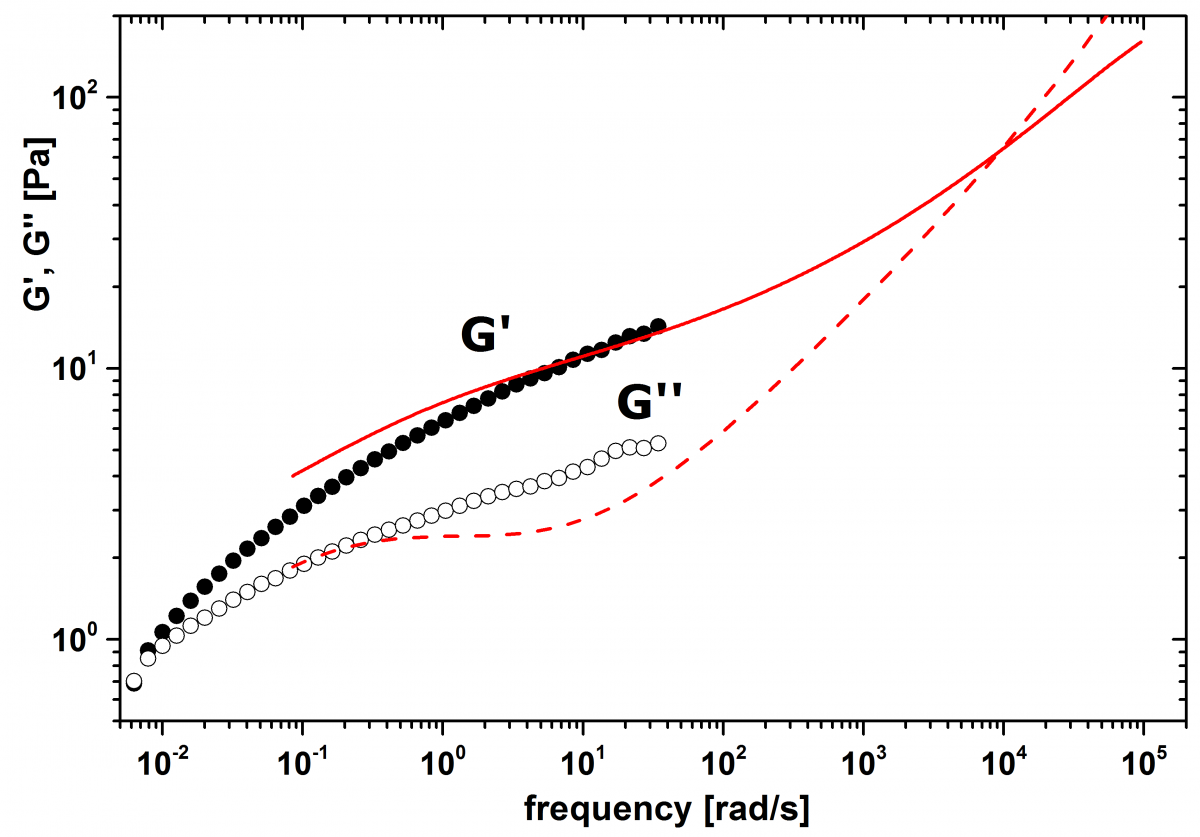
Figure 2. Storage modulus (full symbols, full line) and loss modulus (open symbols, dashed line) of the emulsion. The data obtained from DWS (lines) agree well with the data obtained from the mechanical rheology (symbols).
The prepared emulsion has a moderate volume fraction of the dispersed phase, therefore the rheological properties are mainly determined by the continuous phase. The continuous phase in our model system is a gel with 0.55 wt% xanthan and well known rheological properties [3,4].
We measure the continuous phase separately by DWS microrheology and mechanical rheology. For mechanical rheology, we use the same measurement parameters as for measurements on the emulsion. DWS on the continuous phase is done on a sample prepared by adding polystyrene (PS) particles with diameter 980 nm (dispersed in water) to a xanthan solution as prepared as above but with 0.58 wt.% xanthan. The resulting mixture contained 1.0 wt.% PS particles in a continuous phase with 0.3 wt.% Cosmocil and 0.55 wt.% xanthan. Thus, the PS particles probe an environment corresponding to the continuous phase of the emulsion. DWS on the emulsion is performed in cuvettes with thickness L=2 mm in transmission geometry. For calibration, a dispersion in water of 1.0 wt.% PS particles with a diameter of 980 nm is used. The measured value for the transport means free path l* of the emulsion is 398 µm.
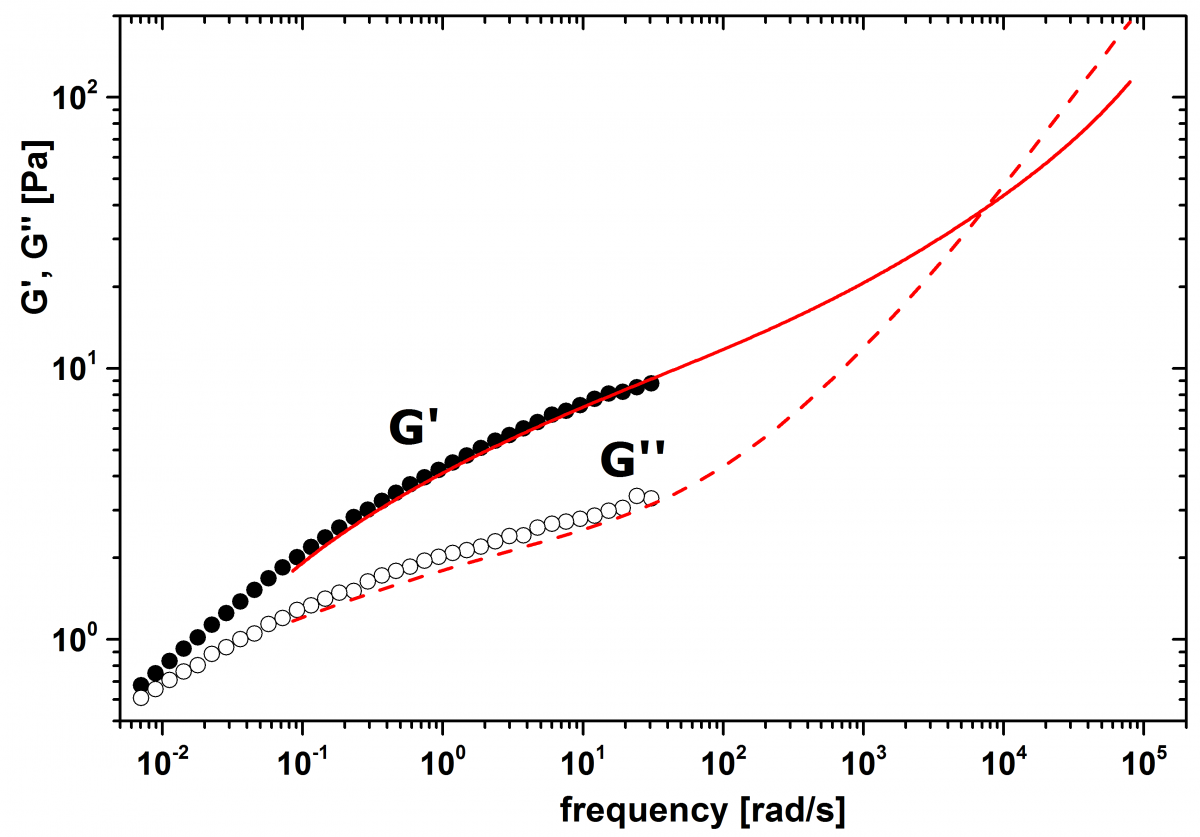
Figure 3. Storage modulus (full symbols, full line) and loss modulus (open symbols, dashed line) of the continuous phase only. The data obtained from DWS microrheology (lines) agree well with the data obtained from mechanical rheology (symbols).
Figure 3 compares the values for G’ and G’’ measured by DWS microrheology and mechanical rheology. In the overlapping frequency range, the data sets show excellent agreement. Moreover, the observed crossover frequency (where G’=G’’) of about 104 rad/s is in very good agreement with literature [4]. Comparing Figure 2 and Figure 3, we find that the moduli of the emulsion are about 50% enhanced compared to the continuous phase whereas the overall shape of the curves is very similar. This confirms the hypothesis that for emulsions with moderate volume fraction the rheological properties are mainly determined by the continuous phase. In a crude approximation, the liquid droplets in an emulsion can be interpreted as suspended solid particles that increase the overall viscosity η with respect to the viscosity of the continuous phase η0 according to the Batchelor equation [5]:
that yields, for a volume fraction φ = 0.2, an increase in G’’(ω) = ηω of 70%. The observed value of 50% is somewhat lower and might be explained by the polydispersity of the droplets that allows better packing.
Conclusion
We applied DWS microrheology to an oil-in-water emulsion and compared the results to mechanical rheology. In the overlapping frequency range, very good agreement between the two techniques was found. Moreover, the continuous phase (xanthan solution) has been characterized separately by DWS microrheology and mechanical rheology and found in excellent agreement with each other and also with literature. This demonstrates that the droplets, i.e. the dispersed phase, can act as tracer particles to probe the rheological properties of the emulsion. Moreover, provided the droplet size is known, we showed that DWS microrheology is well suited for the quantitative characterization of emulsions.
However, care must be taken when the continuous phase of an emulsion is inhomogeneous on the length scale of the droplets. In this case, a quantitative characterization of emulsions is more difficult because the droplets probe a variety of different local environments [6]. In the investigated model system this was not the case since the mesh size of the xanthan network at the chosen concentration is on the order of 40 nm [4], thus well below the droplet size.
Finally, the values obtained by DWS microrheology are highly reproducible and might thus allow the study of time-dependent properties of emulsions such as aging or stability.
References
[1] D.A. Weitz, and D.J. Pine, Diffusing-Wave Spectroscopy. In Dynamic Light Scattering; Brown, W., Ed.; Oxford University Press: New York, 652-720 (1993).
[2] D. Lopez-Diaz, and R. Castillo, Microrheology of solutions embedded with thread-like supramolecular structures, Soft Matter 7, 5926–5937 (2011).
[3] E. Choppe, F. Puaud, T. Nicolai, and L. Benyahia, Rheology of xanthan solutions as a function of temperature, concentration, and ionic strength, Carbohydrate Polymers 82 (2010).
[4] K.v Gruijthuijsen, H. Vishweshwara, R. Tuinier, P. Schurtenberger, and A. Stradner, Origin of suppressed demixing in casein/xanthan-mixtures, Soft Matter 8 (2011).
[5] S.R. Derkach, Rheology on the way from dilute to concentrated emulsions, Inter. Rev. Chem. Eng. 2 (2009).
[6] F.K. Oppong, L. Rubatat, B.J. Frisken, A.E. Bailey and J.R. de Bruyn, Microrheology and structure of a yield-stress polymer gel, Physical Review E 73 (2006).
