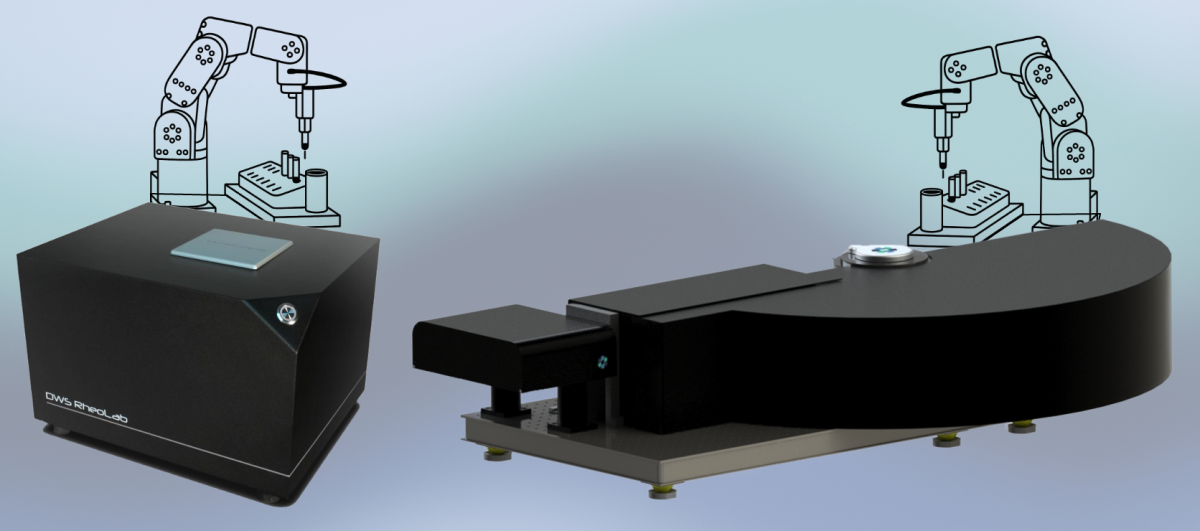
2025/11 - High Throughput option for DWS Rheolab and LS Spectrometer II in 2026
Date: 15.11.2025
LS Instruments Receives First Orders for High-Throughput Robotic Systems for DWS Rheolab and LS Spectrometer II.
Coming in 2026!
Fribourg, Switzerland – October 28th, 2025 — LS Instruments AG has received its first customer orders for robotic high-throughput systems designed for the DWS Rheolab and LS Spectrometer II platforms. These systems, currently in development, are tailor-made and expected to be fully operational by the end of 2025 / beginning of 2026.
From 2026 onward, LS Instruments will offer these automated robotic options as part of its standard product portfolio.
Meeting the Growing Demand for Automation
The introduction of robotic automation addresses the industry’s increasing demand for productivity, reproducibility, and traceability.
Integrating robotic handling with LS Instruments’ DWS and LS platforms enables:
-
50–70% shorter formulation development cycles,
-
Lower analytical costs per condition, and
-
Enhanced regulatory readiness and batch-to-batch comparability.
These improvements are especially impactful for pharmaceutical, biotechnology, materials science, and food research laboratories, where accelerated R&D and data quality are essential for innovation.
A Unique Analytical Platform
By combining the DWS Rheolab and LS Spectrometer II with robotic high-throughput automation, LS Instruments provides researchers with a unique analytical platform for studying microrheology and particle dynamics under fully controlled, repeatable conditions.
Key Benefits for Researchers (R&D Stage)
-
Faster Development Cycles – High-throughput automation enables parallel testing of multiple formulations, cutting R&D time by 50–70%.
-
Higher Data Reproducibility – Automated handling minimizes human error and improves consistency of sample preparation and measurement.
-
Expanded Parameter Exploration – Researchers can test many conditions (temperature, concentration, additives) in one run, enabling more robust optimization.
-
Real-Time Insights – Continuous data acquisition and automated analysis speed up decision-making during formulation screening.
-
Reduced Analytical Costs – More results per run and less manual work lower cost per condition.
-
Better Collaboration and Traceability – Standardized protocols ensure reproducibility between labs and facilitate regulatory documentation.
Key Benefits for Quality Control (QC Stage)
-
Batch-to-Batch Comparability – Automated, identical procedures ensure reliable cross-batch comparison and trend monitoring.
-
Regulatory Readiness – Integrated data logging and traceable workflows simplify GMP and FDA compliance.
-
Reduced Operator Variability – Automation removes subjective influences from manual testing.
-
Higher Throughput in Routine Testing – Multiple samples analyzed in parallel reduce bottlenecks and speed release testing.
-
Predictive Quality Analytics – Early detection of deviations or instabilities supports proactive process control and higher product reliability.
