DLS Microviscometry with the NanoLab 3D: A novel approach to measure viscosity
Related Product
NanoLab 3D™
The NanoLab 3D™ is a compact DLS instrument for particle sizing that is based on the groundbreaking and patented Modulated 3D Cross-Correlation technology. It efficiently suppresses multiple light scattering and therefore sample dilution is no longer required for most samples.
Abstract
In biopharmaceutical development, the reliable and precise measurement of viscosity has always been challenging. Typically, only scarce amounts of sample volume are available, rendering conventional viscometry impractical. The latter typically requires more than one hundred microliters of sample volume with a high biopharmaceutical concentration. 3D DLS Microviscometry is an innovative approach that not only allows the measurement of the smallest volumes (<5 μl) but also allows the measurement of very turbid samples without dilution when combined with the 3D Modulation technique. To demonstrate the advantages of 3D DLS Microviscometry, the viscosity of five different glycerol-water mixtures was measured with a NanoLab 3D, which has been proven in scientific studies to work equally well for proteins [1,2]. A special cell holding as little as 4 μl of sample by volume has been used. The obtained data was analyzed directly by the NanoLab 3D software without any post-processing and was compared to literature values obtained by classic viscometry measurements using ml-sized samples. The perfect agreement shows that DLS Microviscometry offers comparable performance and accuracy on dramatically reduced sample volumes.
What is DLS Microviscometry?
With Dynamic Light Scattering (DLS) one measures the diffusion coefficient of the particles in suspension in a solvent. If one knows the viscosity of the solvent and the sample temperature, one can accurately determine the hydrodynamic radius of the particles from the measured diffusion coefficient (see Appendix for details). This is known as a DLS Sizing experiment and is the most common application of DLS.
Another possible application of this technique is the case in which one wishes to measure the viscosity of a liquid sample, such as a protein solution or a colloidal suspension.
In this case, one can perform a DLS Microviscometry measurement on a sample by adding a small quantity of particles of known hydrodynamic radius (so-called “tracer particles”), such that light scattered from the sample is predominantly caused by these particles. Just as in the case of a regular DLS Sizing experiment one then obtains a highly accurate measurement of the diffusion coefficient, which allows an equally accurate calculation of the sample’s viscosity. The temperature of the sample being the only other required parameter.

Figure 1. 4 µL sample measurable with the NanoLab 3D
Compared to conventional microviscometry, using microfluidic devices, DLS Microviscosimetry measures true zero shear viscosity. Measurement under flow, for example in a capillary, can lead to shear-thinning or thickening and can cause other problems such as shear-induced aggregation in biopharmaceuticals. Moreover, we demonstrate here that the sample volumes required for DLS Microviscosimetry (4 µl) are more than an order of magnitude smaller compared to conventional commercial microviscometry devices, requiring more than 100 µl of sample volume.
Important to note is the fact that DLS Microviscosimetry, just as DLS Sizing, requires the absence of multiple scattering in the signal. In standard DLS experiments, this requirement poses a limit to the maximum allowed tracer particle concentration. This would usually exclude experiments on strongly scattering transparent and/or turbid samples (for example, high concentration protein formulations) where high concentrations of tracer particles are needed to “shadow” the sample signal and ensure that the scattered light is predominantly caused by the tracer particles.
To overcome this limitation, we employ a NanoLab 3D that integrates the 3D Modulation technology (invented and patented by LS Instruments).
The 3D Modulation technology suppresses multiple scattering, thus effectively removing an upper limit to the tracer particle dose, allowing for an error-free determination of the viscosity for all kinds of samples, including those showing substantial turbidity. We illustrate the benefit that 3D Modulation brings to DLS Microviscosimetry experiments on a series of mixtures of water and glycerol.
Sample Preparation
Five milli-Q water-based solutions at different glycerol concentrations (10, 20, 30, 40, and 50 wt. %) were prepared and mixed with 0.006 wt. % of polystyrene particles (107 nm-diameter).
To demonstrate the advantages of 3D DLS Microviscosimetry, the viscosity of five different glycerol-water mixtures was measured with a NanoLab 3D in a special cell holding as little as 4 µl of sample by volume.
Each sample was measured 8 times and each test ran for 45 seconds in the 3D Modulation measurement mode. All measurements were carried out at 20°C, and a waiting time of 30 minutes was applied to the first measurement of each sample series to allow the temperature to stabilize. Each measurement series was completely automated and executed with one click. Despite the low particle concentration, the samples appeared turbid. This means that multiple scattering would have influenced the reliability of the measurements. For this reason, the 3D Modulation mode was chosen, since it suppresses multiple scattering.
Results
Figure 2 shows the autocorrelation function (a) of a single run measurement performed in the Modulated 3D mode and the two channels collecting the intensity fluctuations (b). The NanoLab 3D software converts this raw data into the corresponding viscosity using the DLS Microviscosimetry procedure.
In order to show the reliability and robustness of the results, figure 3 shows all the repetition runs on the same sample. In this example, the related average value of the viscosity was 5.73 ± 0.18 mPa.s.
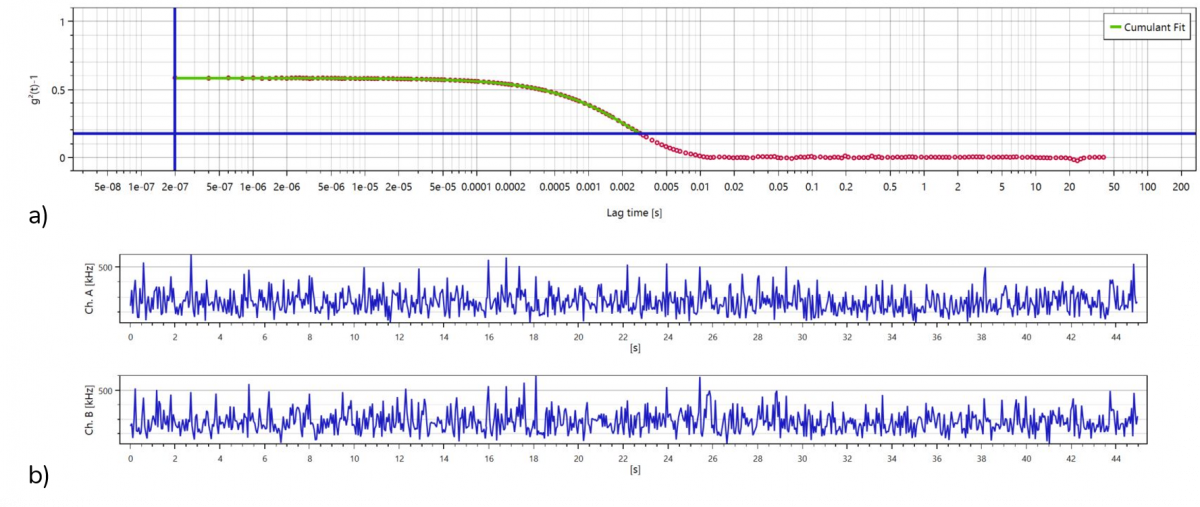
Figure 2. Autocorrelation function (a) and light intensity fluctuation detected through the 2 channels used in the 3D Modulation mode (b)
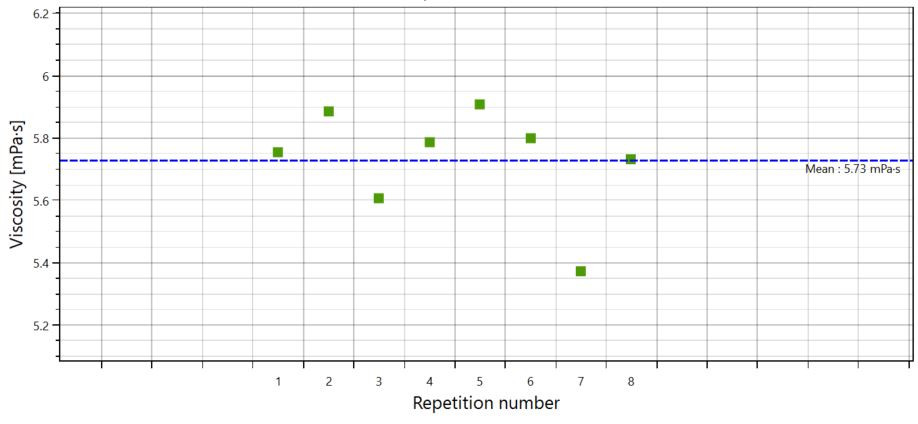
Figure 3. Average viscosity value obtained through 8 repeated measurements
Figure 4 shows the viscosity results of all solutions at different glycerol concentrations obtained by performing DLS Microviscosimetry measurements with the NanoLab 3D.
The results were compared to those obtained with standard viscometers (data available in literature1), showing a perfect match.
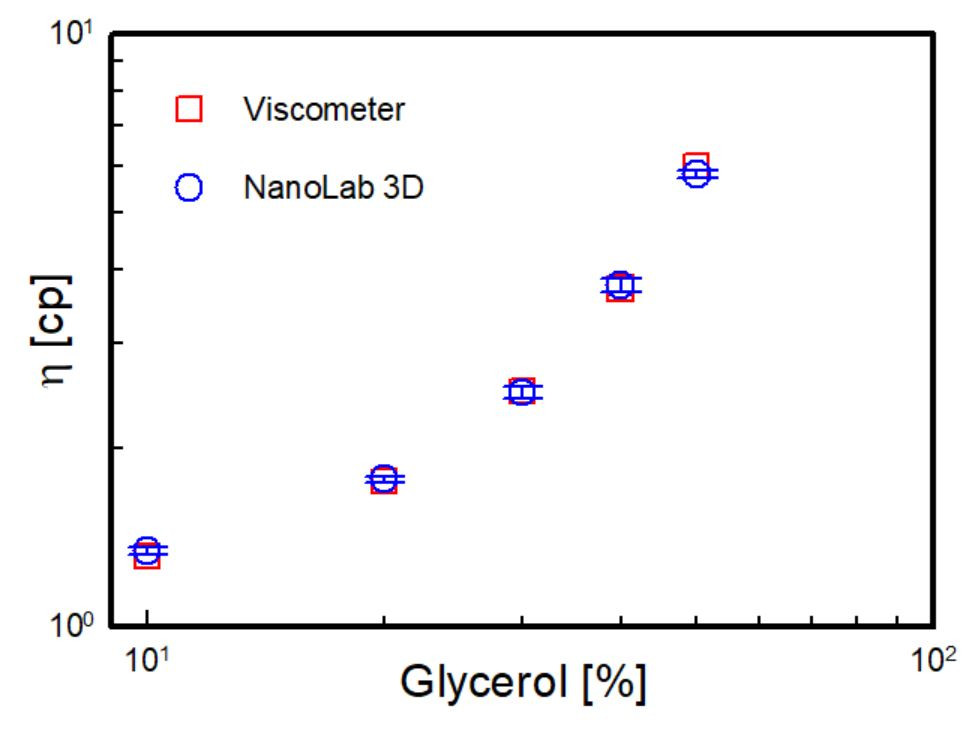
Figure 4. The viscosity of water-glycerol solutions as function of the glycerol mass percentage measured with classic viscometer3 (red squares) and with DLS Microviscosimetry via NanoLab 3D (blue circles, blue segments being the error bars)
The agreement between the classic approach and the results obtained with the NanoLab 3D through DLS Microviscosimetry confirms how powerful this technique is.
Moreover, the common DLS limitation of turbid and/or strongly scattering samples is overcome by the modulated 3D cross-correlation technology which suppresses multiple scattering. This greatly facilitates sample preparation and makes this method suitable for a much wider range of samples.
Appendix
In a Dynamic Light Scattering (DLS) experiment, the Intensity Correlation Function g2(t) is measured at a fixed scattering angle , which in turn defines the scattering vector q=(4
), where
is the refractive index of the sample and
is the wavelength of the incident light. The Field Correlation Function, g1(t) is obtained from the measured Intensity Correlation Function, g2(t) as
(1)
where b is the measured signal intercept. For a monodisperse, Brownian particle system, the Field Correlation Function takes the form
(2)
Equation (2) allows determining the diffusion coefficient 0 from the experimental data, which in turn contains information about the solvent viscosity
and the particles’ hydrodynamic radius
h , given the Stokes-Einstein relation
(3)
where B is Boltzmann’s constant and
is the temperature of the particle suspension.
Knowing h of a tracer particle, obtained for example from a standard DLS sizing experiment, we can determine the viscosity
with the advantages that DLS offers in terms of measurement accuracy, statistical relevance, and sample volume requirements.
Multiple Scattering: Note that other commercial instruments implement a backscattering geometry to reduce contributions from multiple scattering. While this is already a dangerous approach in classical DLS experiments as it does not allow for verification of the amount of residual multiple scattering, this is even more problematic in DLS Microviscometry. Here larger tracer particles are used to have a dominating tracer scattering contribution at concentrations that exclude any systematic errors that would be caused by interaction effects between the tracers. Such particles have however a measurable form factor, i.e. they scatter primarily at smaller scattering angles, and the scattering contribution from the particles measured in a backscattering geometry is thus reduced, while for example scattering from a concentrated protein sample and contributions from multiple scattering are quite independent of the scattering angle. In this case, only the use of 3D DLS guarantees that only singly scattered light is measured and that the resulting microviscometry data is not suffering from unrecognized systematic errors.
References
1. Shire, Steven J., Zahra Shahrokh, and Jun Liu. Journal of pharmaceutical sciences 93.6 (2004): 1390
2. Garting, Tommy, and Anna Stradner. Small 14.46 (2018): 1801548.
3. Glycerine Producers' Association. Physical properties of glycerine and its solutions. Glycerine Producers' Association, 1963.
