Nanomilling and Size Characterization of Highly Concentrated API Suspensions
Joelle Medinger, Coline Bretz, Andrea Vaccaro, Dario Leumann, Xin Gao, Christian Rhême, Marco Lattuada
Related Product
NanoLab 3D™
The NanoLab 3D™ is a compact DLS instrument for particle sizing that is based on the groundbreaking and patented Modulated 3D Cross-Correlation technology. It efficiently suppresses multiple light scattering and therefore sample dilution is no longer required for most samples.
Abstract
Keywords: Active pharmaceutical ingredients, bioavailability, wet nanomilling, DLS, modulated 3D cross-correlation
To increase the bioavailability of active pharmaceutical ingredients (APIs), milling techniques are used to reduce the size of the particles to nanometric size. One technique used for this purpose is nanomilling, in which the particles are ground in a suspension. Highly concentrated conditions, as well as precise process control, are required for a high yield. This requires a reliable particle size analysis that works even with very turbid samples. In this work, we show how suspensions of Naproxen and Fenofibrate can be ground to particle sizes of 79 nm and 173 nm, respectively, using a Frewitt FlexMill-Lab mill equipped with a dynamic separator to separate the milling beads from the API suspension. The size of the ground active ingredients in suspension was measured in native, extremely turbid samples using Modulated 3D Dynamic Light Scattering (DLS) on the NanoLab 3DTM from LS Instruments. The results demonstrate the potential of DLS particle size analysis for quality and process control of nanomilling processes.
Introduction
In the constant research for more efficient drugs which can be administered on target and at patientcompatible concentrations, new interesting molecules have been discovered. Many of those molecules, while presenting high potency, are hydrophobic and consequently difficult to dissolve in the blood. Hydrophobic APIs thus need to be rendered sufficiently water-soluble to travel through the bloodstream, in order to increase their bioavailability. This can be achieved by reducing their size down to the nanometer range (typically below 200 nm) and stabilizing them with biocompatible surfactants [1]. To this end, two approaches are generally employed: in the so-called "bottom-up" approach, the API nanoparticles are generated through agglomeration or granulation of single molecules. This process needs to be interrupted once the target size is reached. However, stopping nucleation and growth is challenging and most often involves the use of biohazardous substances. Additionally, the scalingup of such processes is delicate and laborious. An alternative solution is the "top-down" approach, during which an initially micrometer-sized product is processed to reduce its initial size down to a target size in the nano range [2] . A prominent process for such size reduction is bead milling. A major challenge in the top-down approach is the development of an efficient size reduction process by using a highly performant mill. Additional challenges are the stabilization and characterization of the highly concentrated ground suspensions [3] . Particle size and particle size distribution need to be measured to determine whether the desired milling size has been achieved, whether the stabilization of the particles has worked, and whether no agglomerates have formed [4] . Such characterization is usually performed by means of Dynamic Light Scattering (DLS).
In most wet and dry grinding processes, the lower size limits are well above the nanoscale. When it comes to grinding products to the nano range, current milling methods face problems such as clogging of the sieves due to the small grid size needed for keeping the 200 microns milling beads in the process. Interrupting the process to change or clean the sieves are costly and time-intense intervention. To solve these issues, Frewitt (Fribourg, Switzerland) has launched the innovative FlexMill-Lab NanoWitt. It features a patented and unique dynamic separator that does not depend on beads- or raw product particle size. It enables an easy product and bead loading as well as a highly efficient separation of milling beads from the API suspension. There is no risk of filter clogging requiring replacement or cleaning, which causes process difficulties on conventional nanomills. The NanoWitt technology allows API milling down to a final product size of 50 nm in diameter in a single operation and with a minimal temperature increase. This is achieved with a reduced beads load, and a higher energy efficiency compared to other bead mill equipment on the market. Additionally, the NanoWitt can be adapted to enable at-line sampling for size monitoring of the system through DLS. DLS is preferred as a characterization technique thanks to its in-situ approach and fast measurement time. However, standard DLS is only reliable in the case of highly diluted samples. In samples of moderate to high concentration, a phenomenon called multiple scattering can occur and severely compromise sizing results [5]. Standard DLS cannot detect multiple scattering and hence cannot guarantee the accuracy of the size measured. However, in the context of fast, in-situ monitoring of a milling process, dilution of the sample is not desirable.
Advanced DLS techniques such as the Modulated 3D technology enable complete suppression of multiple scattering, ensuring a full accuracy of the results regardless of the sample concentratio [6] . This technology is implemented in the NanoLab 3DTM from LS Instruments (Fribourg, Switzerland). Removing the need for drastic dilution of the sample enables rapid and straightforward characterization of the particle size and allows for fast feedback on process quality. In addition, the measurement of highly concentrated samples enables realistic stability studies on the product.
Materials & Methods
In this work, two model APIs were used: Naproxen, an anti-inflammatory and antipyretic analgesic, and Fenofibrate, a drug used to treat lipid metabolism disorders by lowering blood cholesterol levels.
The APIs were mixed with the surfactants in distilled water. Pharmacoat 603 (Hydroxypropyl methylcellulose) was purchased from Shin Etsu Chemical Co. Sodium dodecyl sulfate (SDS) and Dioctyl Sulfosuccinate sodium salt (DOSS) were purchased from Sigma Aldrich.
250 mL of a 20% w/w Naproxen formulation were prepared as follows: 7.5 g Pharmacoat, and 0.25 g DOSS were used as surfactants and dissolved overnight in 192.25 g distilled water before 50 g of Naproxen were added to the solution.
250 mL of a 20 % w/w Fenofibrate suspension was prepared by dissolving 0.3 g SDS and 12 g Pharmacoat as surfactants overnight in 228 mL of water before adding 60 g of Fenofibrate.
After the loading of the API suspension into the NanoWitt milling chamber, 70% v/v milling beads were added. Yttrium-coated zirconia beads of 100- 200 µm diameter were used as milling beads. A rotation speed of 7.8 m/s was used in all experiments.
To follow the milling process, 2 mL of sample were taken from the product container after 30, 60, and 120 minutes and every following hour until the milling process was considered finished. Directly after sampling, particle size and particle size distribution were measured with a laser diffraction technique using a Malvern Mastersizer 3000. For this, the milled suspension needed to be diluted at least 1:150 with distilled water. The obtained results were used as a qualitative indication of the milling progress and to detect the end of the API milling process.
Once an optimal milling protocol was established, samples were taken again after 30, 60, and 120 minutes and every following hour until the milling process was finished. Samples were measured 24h later with the NanoLab 3DTM. The samples were diluted with a ratio of 1:4 with distilled water and added to a high-precision optical glass cuvette for measurement. In addition to the Modulated 3D DLS acting as a filter for multiple scattering, the NanoLab 3DTM also provides automated positioning of the cuvette relative to the laser beam and detector. This was used to place the cuvette in a corner position, hence reducing laser path length through the sample and further increasing the highest measurable sample concentration. Finally, the CORENN algorithm was used to extract the particle size distribution (PSD). CORENN is an advanced machine learning algorithm that weights leading approximation techniques and a unique theoretical assessment of the signal noise [7]. In comparison to the commonly used CONTIN or Cumulant method, CORENN can separate particle populations that are close in size and provides solid results that are robust against experimental distortion.
To confirm the DLS measurements, SEM images were taken on a TESCAN Mira 3 LM field emission microscope.
Results & Discussion
Table 1 shows the size evolution for both Naproxen and Fenofibrate samples taken at different times during the milling process and measured 24 h later with the NanoLab 3DTM.
|
API: |
Naproxen |
Fenofibrate |
|
Time (min) |
Diameter (nm) |
|
|
0 |
3410 |
3640 |
|
30 |
365 |
327 |
|
60 |
160 |
247 |
|
120 |
150 |
214 |
|
180 |
120 |
196 |
|
240 |
85 |
173 |
|
300 |
79 |
- |
Table 1 Average diameter measured at different times during the NanoWitt milling process for the two model APIs using the NanoLab 3DTM .
The milling process of Naproxen was stopped after 5 hours once a target particle size of 80 nm was reached. We note that to measure the DLS particle size of Naproxen, a laser power of 30 mW was necessary to generate a sufficiently high signal, due to the high sample concentration. The size of the Naproxen particles with proceeding milling time is represented in Figure 1.
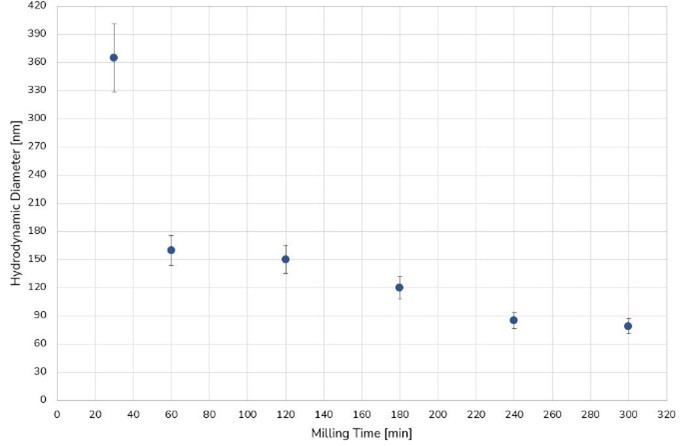
Figure 1. Size evolution of Naproxen as a function of milling time (minutes).
The size of the particles was continuously reduced over time until a plateau was reached. While a fast size reduction can be observed in the first hour of milling, it is followed by a slower decrease until the final particle size of ~80 nm was reached. The PSD obtained through the CORENN algorithm reveals one single particle population at the end of the milling process as can be seen in Figure 2.
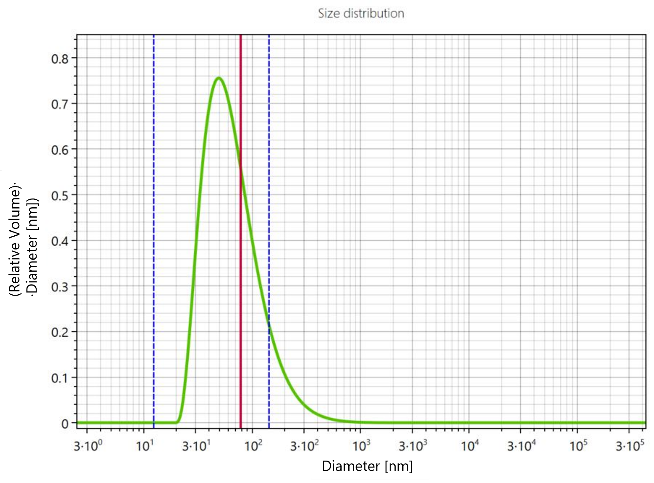
Figure 2. PSD of the Naproxen suspension after 300 minutes of milling.
The presence of a single particle size and the fact that the measurement was taken 24 h after the milling process was completed suggests that the suspension was stable. However, it should be noted that for a shelf-life study and a more meaningful statement on the stability of the system, significantly more data points at larger storage time intervals are required. 4 APPLICATION NOTE To confirm the DLS sizing results, SEM images were acquired. The SEM images, shown in Figure 3, confirm the final particle size measured by DLS. A particle shape variation from spherical to slightly rod-shaped Naproxen particles can also be observed.
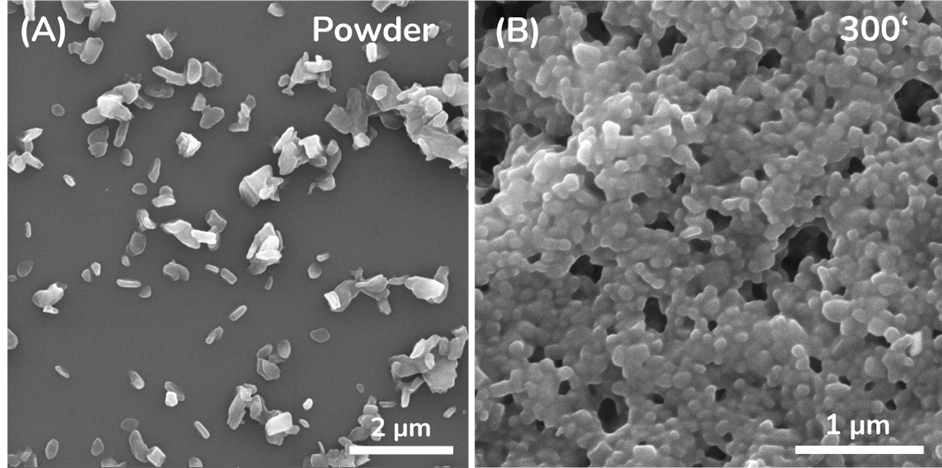
Figure 3. SEM images of Naproxen (A) initial powder and (B) final nanosized product after the milling process.
A possible explanation for the difference in particle shape could be the crystal growth of the API particle promoted by the external energy transmitted by the milling beads. However, in our case, the change in shape is minimal and will not falsify the message provided by the DLS measurements.
Fenofibrate was milled for 4 hours. After 2 hours a particle size close to the final 170 nm in diameter was already reached, as can be seen in Figure 4.
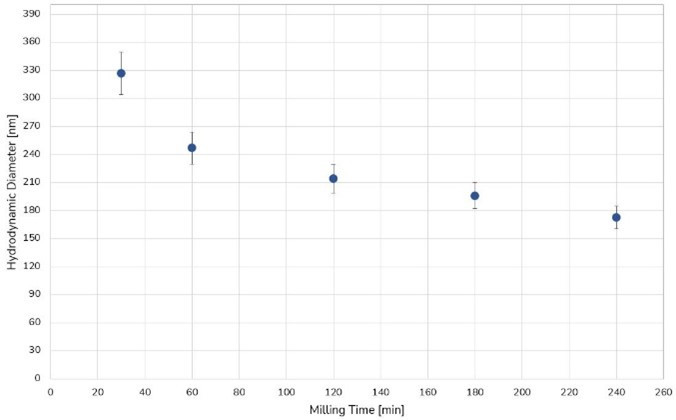
Figure 4. Size evolution of Fenofibrate over time.
The DLS measurement of the Fenofibrate samples required even higher laser power compared to Naproxen. A laser power of 50 mW was required to generate a sufficiently high signal. This shows that laser power also plays an important role in the accurate characterization of concentrated and turbid samples.
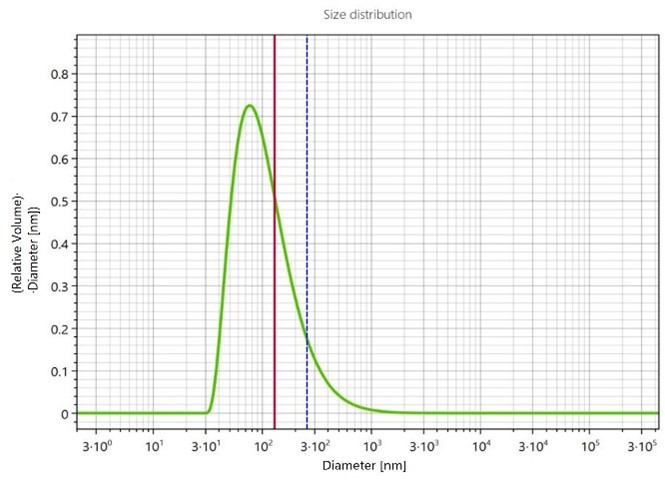
Figure 5. PSD of the Fenofibrate suspension after 240 minutes of milling.
A single particle population of 170 nm in diameter was detected at the end of the milling process as illustrated by the PSD shown in Figure 5. This suggests, analogous to the Naproxen sample, that no agglomerates have formed, and the formulation can hence be considered stable after 24h.
 Figure 6. SEM images of Fenofibrate (A) initial micronized powder and (B) final nanosized product after the milling process.
Figure 6. SEM images of Fenofibrate (A) initial micronized powder and (B) final nanosized product after the milling process.
In the case of Fenofibrate as well, SEM images were recorded to visually confirm the final particle size and to show the particle shape after the milling process. From the picture reported in Figure 6, it can be seen that the particle shape is not perfectly spherical. However, the particle shape is close enough to spherical to consider the size measurement by DLS trustworthy.
Conclusion
In this work, we show that two model APIs - Naproxen and Fenofibrate - were successfully milled down to nanosizes (79 nm and 173 nm, respectively) by using the Frewitt Flexmill-Lab Nanowitt. Using advanced DLS, meaningful results for particle size and particle distribution of milled APIs in suspension were obtained, which can be used to determine the stability of the product but also support the setup of the milling process. The samples could be measured at high concentration using the NanoLab 3DTM. The use of the Modulated 3D technology, as also the high laser power, enabled measuring samples in a concentration close to the typical concentrations found in industrial API nanomilling processes. The results show that by adjusting the API concentration in suspension, a process with high concentration and yield could be developed with real-time process control by combining the NanoWitt with the NanoLab 3DTM. The potential cost reductions generated in the production of highly bioavailable APIs as well as the improved quality control can ultimately benefit the production of custom formulations.
Authors
Joelle Medinger, Coline Bretz, Andrea Vaccaro, Dario Leumann, Xin Gao, Christian Rhême, Marco Lattuada
References
[1] Pandey, P.; Gulati, N.; Makhija, M.; Purohit, D.; Dureja, H. Nanoemulsion: A Novel Drug Delivery Approach for Enhancement of Bioavailability. Recent Pat. Nanotechnol., 2020, 14 (4), 276–293.
[2] Bitterlich, A.; Laabs, C.; Busmann, E.; Grandeury, A.; Juhnke, M.; Bunjes, H.; Kwade, A. Challenges in Nanogrinding of Active Pharmaceutical Ingredients. Chem. Eng. Technol., 2014, 37 (5), 840–846.
[3] Lee, J.; Choi, J. Y.; Park, C. H. Characteristics of Polymers Enabling Nano-Comminution of Water-Insoluble Drugs. Int. J. Pharm., 2008, 355 (1–2), 328–336.
[4] Knieke, C.; Azad, M. A.; Davé, R. N.; Bilgili, E. A Study of the Physical Stability of Wet Media-Milled Fenofibrate Suspensions Using Dynamic Equilibrium Curves. Chem. Eng. Res. Des., 2013, 91 (7), 1245–1258. 3.02.008.
[5] Urban, C.; Schurtenberger, P. Characterization of Turbid Colloidal Suspensions Using Light Scattering Techniques Combined with CrossCorrelation Methods. J. Colloid Interface Sci., 1998, 207 (1), 150–158. https://doi.org/10.1006/JCIS.1998.5769.
[6] Block, I. D.; Scheffold, F. Modulated 3D Cross-Correlation Light Scattering: Improving Turbid Sample Characterization. Rev. Sci. Instrum., 2010, 81 (12), 123107.
[7] https://lsinstruments.ch/en/theory/dynamiclight-scattering-dls/dls-data-analysis-thecorenn-method.
