Experimental guidelines - Sample preparation
Diffusing Wave Spectroscopy (DWS) is often considered an extension of Dynamic Light Scattering (DLS). While DLS is based on single light scattering, DWS analyses light that has been scattered multiple times. As a consequence, DWS samples are highly turbid and are typically white. DWS is primarily applied for microrheology, but can also be used for particle sizing.
I) Samples for DWS microrheology
In a typical DWS microrheology experiment, the motion of scattering centers (here called tracers) is measured and analyzed in order to characterize the rheological properties of the sample.
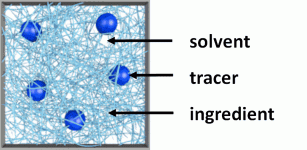
Figure 1. Typical DWS microrheology sample where we can identify the tracers, and the ingredients dissolved in the solvent.
Figure 1 depicts a typical DWS microrheology sample where we can identify tracers that scatter the probing light and the ingredients in a solvent that determine the rheological properties of the sample. Tracers are responsible for the turbid appearance of the samples and might be naturally part of the sample (e.g. droplet phase in the case of dilute emulsions) or added during the sample preparation (examples in the list below marked with *).
To qualify for a standard DWS microrheology sample the two conditions must be fulfilled:
- the presence of the tracers does not significantly affect the rheological properties of the sample
- the scattering of the tracers is dominant over the scattering caused by the ingredients
Typically, these conditions can be met by choosing a proper concentration of the tracers. The generic picture of the DWS microrheology sample shown in Figure 1 applies to most DWS microrheology experiments and covers systems such as:
- dilute emulsions (<50% volume fraction of the droplet phase)
- polymer solutions*
- protein solutions*
- polysaccharide solutions* (e.g. Xanthan)
- surfactant solutions* (e.g. worm-like micelles)
- gels* (e.g. cross-linked polymer)
II) Samples for other DWS applications
DWS also allows measurements on different types of samples that only partially match with the illustration shown in Figure 1 and are not necessarily aiming at the determination of rheological properties. Prominent examples are the following:
- particles in solvents
The particles (which represent the tracers as defined above) probe the rheological properties of the surrounding solvent. This allows for example measurements of the viscosity of Newtonian solvents. Alternatively, the particle size can be determined in a solvent with known viscosity. Note that at high particle concentrations (>>10%) collective effects might be present in the measured data. - concentrated emulsions (>50% volume fraction of the droplet phase)
The motion of the droplets is restricted due to the high concentration and affected by non-Brownian dynamics like structural relaxation processes. As a consequence, the data analysis of the measured raw data requires expert knowledge.[1] - gels formed from scattering particles (e.g. yogurt)
Here, the particles form the gel and thus determine both the rheological and scattering properties of the sample. - foams
Here, the scattering occurs at the liquid-gas interface of the foam. The measured dynamics in a foam is typically dominated by structural relaxations and data analysis requires expert knowledge.
III) Tracer particles
Transparent or semi-transparent systems (marked with * in the lists above) require the addition of tracer particles. The important aspects to be considered are the following:
- chemical composition
The most common tracer particles used are latex (polystyrene, PS), titanium dioxide (TiO2), and silica (SiO2). The choice of tracer particles depends on the sample one intends to measure. In the case of water-based systems, PS particles with a diameter in the range from 200 to 600 nm are often the best choice. This is due to their stability, monodispersity, and compatibility with many samples. In the case of non-water based systems, the stability of PS particles should be verified. PS beads can be purchased directly from LSI. Other suppliers offer a wide range of particles (e.g. Microparticles GmbH Berlin).
Make sure that your chosen tracer particles are well dispersed and do not undergo any transformation in your system (e.g. swelling or even dissolution of polymeric particles in some organic solvents). - particle size
When selecting the size of your tracer particles keep in mind that they should be adapted to the size dimensions present in your specific system. Consider for example a gel network in a solvent; if the size of the tracer particles is much smaller than the typical mesh-size of the network, you will not measure the rheological properties of the gel, but those of the solvent. Furthermore, the scattering properties of a particle depend on the particle size; for red light the range of 200 to 600 nm features a maximal scattering versus volume ratio, thus one needs to add only small amounts in order to ensure sufficient turbidity for DWS. Please refer also to this answer of the Support FAQ. And last but not least, the larger the particle size the higher tendency towards sedimentation. - concentration
The appropriate concentration of tracer particles needs to be determined before the experiment. In general, the concentration has to be chosen such that the sample turbidity, expressed by the transport mean free path l*, fulfills the condition: L/l* > 7. As a guideline, in the case of monodisperse polystyrene particles of any size in the range of 200-600 nm in diameter used as tracers in an aqueous sample, you should have approx. 1 vol% of particles to obtain sufficient scattering in a cuvette of 2 mm thickness. Follow these simple instructions to obtain 1 g of "DWS" suitable sample:
- Prepare 0.9 g of your transparent sample.
- Then add 0.1 g of polystyrene tracer particles (at 10 vol% concentration).
- Stir well (e.g. by shaking or using a magnetic stirrer) to obtain a good dispersion. If the sample allows you might also sonicate.
Note that the solvent of the added tracer particles dilutes your sample slightly. You might compensate for this effect by preparing your initial sample at an accordingly increased concentration. Finally, check that the obtained transport mean free path l* is 7 times smaller than the thickness of the cuvette (L/l* > 7), otherwise increase tracer particle concentration or use a thicker cuvette. Note also, that an approximate value for l* can be calculated previously using the mie-Calculator which might help you with the planning of a new experiment. - density
Make sure your tracer particles do not sediment during the measurement time. In general, the more viscous sample, the slower sedimentation. Strongly structured samples like gels allow as well the use of very dense particles (e.g. TiO2). - refractive index
Relevant for the scattering behavior of tracer particles is the difference between the refractive indices of the particles and the solvent. When the difference between refractive indices is small, as in the case of PMMA (1.49) and cyclohexane (1.42), an increased concentration of particles is needed to guarantee L/l* > 7. Please refer also to this answer of the Support FAQ. - solvent
Samples used for DWS should be in suspension, meaning that particles are dispersed in the liquid phase. In principle, there are no restrictions regarding the solvent, but the most common are:
- Deionized water or MiliQ water
- Oil
- Toluene
- Methanol
- Ethanol
- Glycerol
- DMSO
IV) Cuvette
Make sure that cuvettes you are using for the measurements are clean and dust-free. Glass and quartz cuvettes can be cleaned with Hellmanex III from Helma. Upon hot sonication, rinse multiple times with pure solvent (for example MiliQ water or absolute ethanol), dry in a dust-free environment (laminar-flow hoods might be advisable when available). For faster drying, an oven at 60°C, vacuum, or N2 can be used. In this case, hold the cuvettes upside down to avoid any contamination.
While pouring suspension into the cuvette, make sure that no air bubbles are introduced. If this happens, sonicate the cuvette or resuspend the suspension with the pipette. To avoid bubble formation, it is good practice to pour the suspension onto the wall of the cuvette.
The optimal filling height is 15 to 20 mm measured from the bottom of the cuvette. The higher filling might lead to thermal convection due to the thermal gradient. A lower filling can introduce artifacts in your measured data because of none conform illumination of the sample.
If you use markers to write on the cuvette, make sure that you do not write on the wall through which the beam traverses.
Before inserting the cuvette into the sample holder, clean it again with a tissue. Make sure that cuvette external walls are dried.
LSI offers 4 sizes of cuvettes: 1 mm, 2 mm, 5 mm, and 10 mm. This allows a more flexible choice of the sample concentration: thinner cuvettes (shorter optical path) for more turbid samples, thicker cuvettes (longer optical path) for less turbid samples.
V) Equilibration
After inserting the cuvette with the sample into the sample holder, wait 10-15 minutes so that the temperature of the sample is the same as the temperature of the thermal chamber. Note also that some samples require a significantly longer equilibrium time because the stress-induced from filling the sample into the cuvette needs to relax. This effect is called thixotropy and is common for highly viscoelastic samples.
References
[1] T.G. Mason, New fundamental concepts in emulsion rheology, Current Opinion in Colloid & Interface Science 4 (1999), 213-238
