Drug Release in Micellar Surfactant
Related Product
DWS RheoLab™
The DWS RheoLab™ is a contact-free rheometer. It provides access to the sample's viscoelastic properties over an unmatched frequency range and enables the study of textures and microstructures while requiring only small sample volumes.
Summary
Understanding the drug release process represents a key step to design and optimize possible innovative carriers. In this application note we present a study on the drug release of the commercial medicine Aspirin®(acetylsalicylic acid, from Bayer, Germany) in a well-known surfactant system based on Cetylpyridinium Chloride, used as carrier. The process was monitored by following the time evolution of the viscoelastic properties of the sample by means of DWS microrheology.
Introduction
Diffusing Wave Spectroscopy (DWS) is a light scattering technique [1,2] that enables the user to perform a quick and accurate microrheological analysis of a viscoelastic fluid sample. It provides information about the medium’s linear rheological properties, namely the frequency-dependent storage and loss moduli, G’(ω) and G’’(ω), respectively and, consequently, the complex viscosity, η* [3]. DWS can be applied to a rich variety of soft-matter systems, whose properties can be analyzed and monitored, and is particularly useful to follow time-dependent processes due to the non-destructive, quick measurements. The possibility to monitor a drug release process using the non-invasive DWS microrheology, over a broad range of frequencies, can be of particular interest in the context of the design and optimization of the release mechanism.
In this work, we monitor the release of commercial Aspirin®in an aqueous surfactant solution. The Aspirin®concentration, like other non-steroidal anti-inflammatory drugs (NSAIDs) [4,5], plays a crucial role in the formation of the micellar microstructure and the dynamics of the overall system. In the past, the influence of the concentration of other NSAIDs has only been studied by manually changing its amount in the suspending medium. Owing to the superior time resolution and the fact that the sample can be kept neatly sealed in a cuvette, we demonstrate that DWS can follow the dissolution process of Aspirin® in a single sample.
During dissolution the Aspirin® concentration increases and changes the rheological properties of the suspending medium. We monitor this process by following the evolution through DWS microrheology. In this way, neither evaporation nor environmental contamination can affect the measurements. The results illustrate the benefits of DWS based microrheology for the study of delivery and release of drug molecules.
Sample preparation
Half an effervescent tablet of commercial Aspirin®-C (Bayer), corresponding to a total amount of 95 mM of acetylsalicylic acid, was dissolved in 10ml of water. The solution was degassed and 100 mM of Cetylpyridinium Chloride was added later.
To perform DWS microrheology, 522 nm-diameter polystyrene particles (microparticles GmbH, Germany) were added to a final concentration of 1% w/w corresponding to a transport mean free path l* of roughly 400 μm. 0.3 mL of solution was filled into a 2 mm path length glass cuvette and sealed to avoid evaporation. The cuvette was subsequently loaded in a DWS RheoLab instrument from LS Instruments. The drug release process was followed at 25°C with an automated measurement script that acquired a normalized intensity correlation function, g2(t)-1, every hour. For reasons of clarity, only some of the results are shown.
Results and Discussion

Figure 1. a) g2(t)-1 and b) MSDs of the micellar solution measured at different times during drug release. Time in hours (see legend).
Figure 1a shows the time evolution of g2(t)-1 of the micellar solution, containing the tracer beads, as the Aspirin® dissolves. A change in the dynamics of the system is observable from the correlation functions of the sample at different times (see the legend in hours): while at the beginning of the experiment the correlation function is characterized by a single decay, a double decay appears at a longer time. Moreover, after about 100 hours, the decay shifts again towards shorter lag times.
In Figure 1b, the corresponding evolution of the Mean Squared Displacements, MSDs, is presented. The transition from the single decay to the double decay correlation function corresponds to the dynamics evolution from a pure viscous (straight MSDs lines) to a viscoelastic behavior (MSDs curves with an intermediate plateau). The non-monotonic trend of the dynamic evolution can also be seen in Figure 1b.
From the MSDs, rheological properties were calculated using the data analysis package delivered with the DWS RheoLab. The evolution of the complex viscosity, η*, as a function of the angular frequency ω as the Aspirin® dissolves is presented in Figure 2a. From the Newtonian plateau of each complex viscosity curve, we can determine the zero-shear rate viscosity, η0, and follow its evolution in time (Figure 2b).
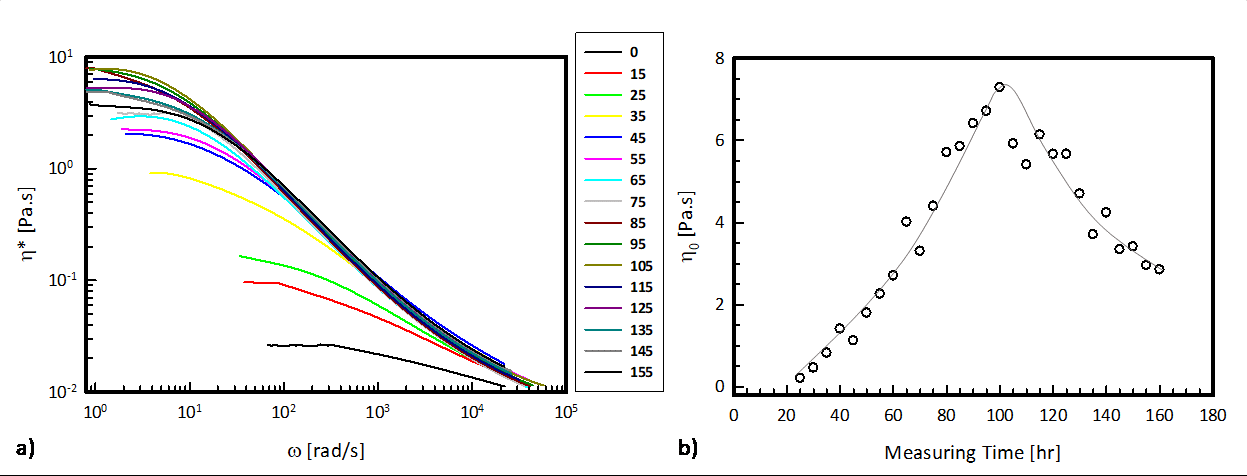
Figure 2 .a) Complex viscosity, η*, as a function of the angular frequency, ω, at different measuring times (see legend in hours); b) Zero-shear rate viscosity, η0, as a function of the measuring time.
The results show a non-monotonic trend of the zero-shear rate viscosity as a function of time, confirming the observations above. Thus, as the Aspirin® dissolves and its concentration in the systems increases, the viscosity increases by almost three orders of magnitude. These results are in agreement with those available in literature for similar micellar systems, where the viscosity is changed by increasing the concentration of NSAIDs [4,5]. Thus, the recorded evolution of the complex viscosity presented in this work can be attributed to morphological changes of the micellar structure.
Conclusion
Using a DWS RheoLab, we have successfully monitored the release of the commercial NSAID, Aspirin®, in an aqueous surfactant solution, via the rheological changes induced by the increasing concentration of the drug in the suspending medium.
The good agreement of our results with those of surfactant micellar structural changes induced by NSAIDs available in literature highlights the high potential of DWS microrheology as a novel technique to monitor and analyze drug release processes. This will facilitate the design of new innovative carriers.
References
[1] D. J. Pine, D.A. Weitz, P.M. Chaikin, E. Herbolzheimer, Diffusing wave spectroscopy, Physical review letters, 1988, 60.12, 1134.
[2] G. Maret, P. E. Wolf, Multiple light scattering from disordered media. The effect of Brownian motion of scatterers, Zeitschrift für Physik B Condensed Matter, 1987, 65.4, 409-413.
[3] E. M. Furst, T. M. Squires, Microrheology, Oxford University Press, 2017.
[4] T.G. Mason, D.A. Weitz, Optical Measurements of Frequency-Dependent Linear Viscoelastic Moduli of Complex Fluids, Phys. Rev. Lett., 1995, 74, 1250-1253.
[5] R. Pasquino, B. De Gennaro, D. Gaudino, N. Grizzuti, On the Use of Nonsteroidal Anti-Inflammatory Drugs as Rheology Modifiers for Surfactant Solutions, Journal of Pharmaceutical Sciences, 2017, 106.11, 3410-3412.
[6] R. Pasquino, M. Di Domenico, F. Izzo, D. Gaudino, V. Vanzanella, N. Grizzuti, B de Gennaro, Rheology-sensitive response of zeolite-supported anti-inflammatory drug systems, Colloids and Surfaces B: Biointerfaces, 2016, 146, 938-944.
